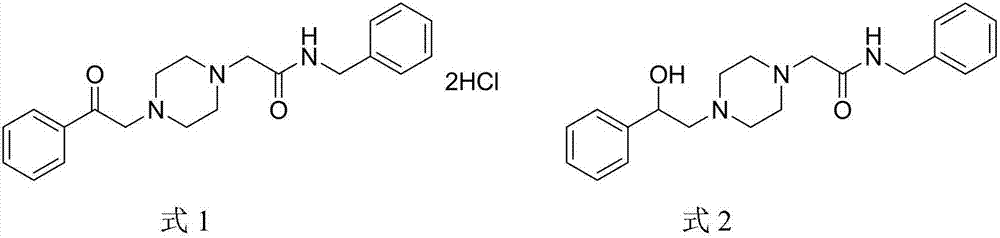
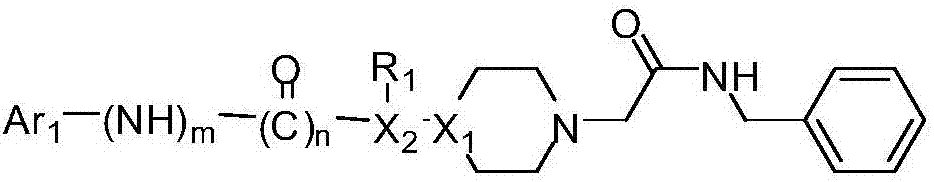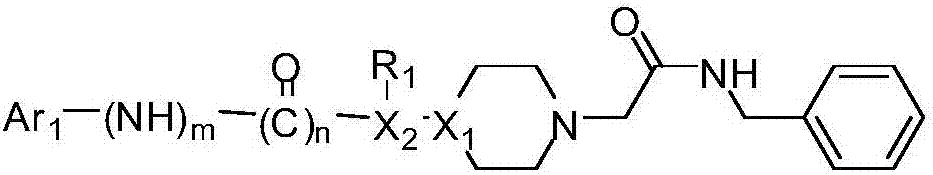Acetylbenzylamine piperazine derivatives and their application as neuroprotective agents
A technology of acetylbenzylamine piperazine and its derivatives, which is applied in the field of acetylbenzylamine piperazine derivatives, which can solve the problems of low activity, difficulty in penetrating the blood-brain barrier, and high cardiotoxicity, and achieve high activity and significant deficiency tolerance. Oxygen activity and less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] N-Benzyl-2-(4-{2-[(5-methyl-1,3,4-thiadiazol-2-yl)amino]-2-carbonylethyl}piperazin-1-yl) Acetamide (V-1) and its hydrochloride
[0083] Put 2-amino-5-methyl-3,4-diazathiophene (20mmol), chloroacetyl chloride (24mmol), and triethylamine (30mmol) in 25ml of acetonitrile, react at room temperature for 8h, follow General Method 2 N-(5-methyl-3,4-diazathiophene)-2-chloroacetamide was obtained with a yield of 67%.
[0084] The above-obtained N-(5-methyl-3,4-diazathiophene)-2-chloroacetamide (12.8mmol) and N-acetylbenzylamine piperazine (II) intermediate (19.6mmol) were put into 30ml acetone solution, add K 2 CO 3 (19.6mmol), KI (6.4mmol), reacted at room temperature for 8h. The insoluble matter was removed by filtration, and the filtrate was concentrated under reduced pressure to obtain a crude product. The crude product was subjected to silica gel column chromatography to obtain the free base N-benzyl-2-(4-{2-[(5-methyl-1,3,4-thiadiazol-2-yl)amino]-2-carbonyl ethyl Bas...
Embodiment 2
[0089] [4-(2-Benzylamino-2-carbonylethyl)piperazin-1-yl]-N-phenylpropanamide (V-2) and its hydrobromide
[0090] Put aniline (20mmol), 2-chloropropionyl chloride (24mmol), and triethylamine (30mmol) in 25ml of acetonitrile, react at room temperature for 8h, and follow the operation of General Method 2 to obtain N-aniline-2-chloropropane Amide, yield 66%.
[0091] Put the N-aniline-2-chloropropionamide (12.8mmol) obtained above and the N-acetylbenzylamine piperazine (II) intermediate (19.6mmol) into 30ml of acetone solution, and add K 2 CO 3(19.6mmol), KI (6.4mmol), reacted at room temperature for 8h. The insoluble matter was removed by filtration, and the filtrate was concentrated under reduced pressure to obtain a crude product. The crude product was subjected to silica gel column chromatography to obtain the free base [4-(2-benzylamino-2-carbonylethyl)piperazin-1-yl]-N-phenylpropanamide (V-2), 4.0g, yield 81.87%.
Embodiment 3
[0096] 2-[4-(2-Benzylamino-2-carbonylethyl)piperazin-1-yl]-N-(4-methoxyphenyl)propionamide (V-3) and its sulfate;
[0097] Put p-methoxyaniline (20mmol), 2-chloropropionyl chloride (24mmol), and triethylamine (30mmol) in 25ml of acetonitrile, react at room temperature for 8h, and follow the operation of General Method 2 to obtain N-p-methyl Oxyaniline-2-chloropropionamide, yield 64%.
[0098] The above-obtained N-p-methoxyaniline-2-chloropropionamide (12.8mmol) and N-acetylbenzylamine piperazine (II) intermediate (19.6mmol) were put into 30ml of acetone solution, and K 2 CO 3 (19.6mmol), KI (6.4mmol), reacted at room temperature for 8h. The insoluble matter was removed by filtration, and the filtrate was concentrated under reduced pressure to obtain a crude product. The crude product was subjected to silica gel column chromatography to obtain free base 2-[4-(2-benzylamino-2-carbonylethyl)piperazin-1-yl]-N-(4-methoxyphenyl)propionamide (V -3), 3.4g, yield 64.63%.
[0099] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com