Derivative of tetraphenyl silicane and dibenzothiophene and method for preparing derivative
A technology of dibenzothiophene and tetraphenylsilicon is applied in the field of organic electroluminescence display, which can solve the problem of rare hole transport materials, improve electron injection/transport capability, and reduce intermolecular agglomeration and interaction. , the effect of good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
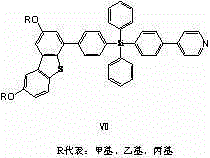
[0026] Example 1, a derivative of tetraphenylsilicon and dibenzothiophene, the derivative has the following structure:
[0027] , the derivative has hole transport properties and can be used for OLED display; in this embodiment, R is methyl, and the structure of the derivative is as follows:
[0028]
[0029] Above-mentioned derivative adopts following steps to prepare:
[0030]
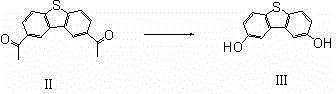
[0031] Wherein the preparation of compound I:
[0032]
[0033] Under the protection of argon, add 14.87g of 1,4-dibromobiphenyl and 210mL of tetrahydrofuran to the three-necked flask successively, cool to -78°C, add 26.25mL of n-butyllithium dropwise, and stir at -78°C for 1 After 1 hour, slowly add 7.59 g of dichlorodiphenylsilane dropwise. After the dropwise addition, the temperature is automatically raised after 1 hour of heat preservation reaction, and the reaction is carried out overnight. Add water to quench the reaction, distill off the solvent, add dichloromethane and water to d...
Embodiment 2
[0057] Example 2, a derivative of tetraphenylsilicon and dibenzothiophene. In this example, R is ethyl, and the structure of the derivative is as follows:
[0058]
[0059] The preparation method of above-mentioned derivative is as follows:
[0060]
[0061] Derivative VII-2 refers to the preparation process of Example 1, except that methyl iodide in the fourth step is changed to ethyl bromide. Derivative VII-2: 1 HNMR (400MHz, CDCl 3 )δ=8.69(dd,J=4.5,1.6Hz,2H,Ar-H),8.24–8.10(m,2H,Ar-H),7.89–7.73(m,7H,Ar-H),7.74–7.62 (m,6H,Ar-H),7.62–7.40(m,12H,Ar-H),3.98(m,4H,OCH 2 -H), 1.33(m,6H,Me-H); LC-MS (ESI): 683[M-H] - , element analysis measured value (calculated value) / %: C79.03 (79.01), H5.45 (5.47), N2.05 (2.03), O4.68 (4.70), S4.69 (4.70), Si4.11 (4.10).
[0062] Melting point: 291°C, glass transition temperature: 118°C, decomposition temperature: 435°C
[0063] Absorption spectrum: λmax=282nm
[0064] Fluorescence spectrum: λmax=378nm
[0065] Hole Mobility: 4.0×1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com