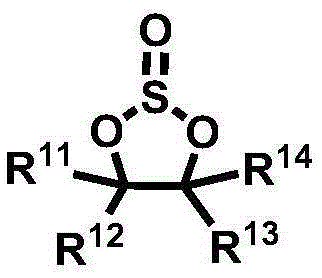
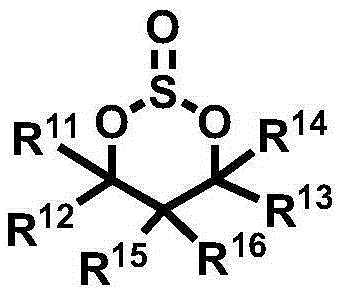
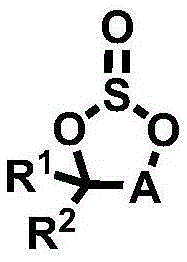Electrolyte for Lithium Secondary Battery and Lithium Secondary Battery Containing the Same
A secondary battery and electrolyte technology, applied in the field of lithium secondary battery electrolyte and lithium secondary battery containing the same, can solve the problems of high volatility and high flammability, and achieve improved discharge Capacity, excellent life characteristics, the effect of improving the capacity recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] [Example 1] (4R,5R)-1,3,2-diazolethiophene-4,5-dicarboxylic acid dimethyl ester 2-oxide (dimethyl(4R,5R)-1,3,2-dioxathiolane- 4,5-dicarboxylate2-oxide, hereinafter also referred to as 'PEA96') synthesis
[0114] In a 100ml round bottom flask, 3.56g of dimethyl-L-tartrate (dimethyl-L-tartrate, 20mmol) and 3.96g of pyridine (pyridine, 50mmol) were dissolved in 40ml of dichloromethane, and then The temperature dropped to 0°C. Under a nitrogen atmosphere, 2.86 g of thionyl chloride (24 mmol) was slowly injected for 30 minutes, and then the temperature was raised to normal temperature. After reacting at normal temperature for 15 minutes, 30 ml of distilled water was added to complete the reaction. After separating the organic layer and the aqueous layer, the aqueous layer was extracted again with 20 ml of dichloromethane, thereby collecting the organic layer, and, for the organic layer, after washing once with 20 ml of 1N HCl solution, washed with 20 ml of saturated sodium...
Embodiment 2
[0116] [Example 2] (4R,5R)-1,3,2-dioxathiophene-4,5-dicarboxylic acid diethyl ester 2-oxide (diethyl(4R,5R)-1,3,2-dioxathiolane -4,5-dicarboxylate2-oxide, hereinafter also referred to as 'PEA97') synthesis
[0117] In a 100ml round bottom flask, 4.12g of diethyl-L-tartrate (diethyl-L-tartrate, 20mmol) and 3.96g of pyridine (pyridine, 50mmol) were dissolved in 40ml of dichloromethane, and then The temperature dropped to 0°C. Under a nitrogen atmosphere, 2.86 g of thionyl chloride (24 mmol) was slowly injected for 30 minutes, and then the temperature was raised to normal temperature. After reacting at normal temperature for 15 minutes, 30 ml of distilled water was added to complete the reaction. After separating the organic layer and the aqueous layer, the aqueous layer was extracted again with 20 ml of dichloromethane, thereby collecting the organic layer, and, for several layers, after washing once with 20 ml of 1N HCl solution, washed with 20 ml of saturated sodium bicarbon...
Embodiment 3
[0119] [Example 3] (4R,5R)-1,3,2-Dioxazolethiophene-4,5-dicarboxylic acid diisopropyl ester 2-oxide (diisopropyl(4R,5R)-1,3,2- dioxathiolane-4,5-dicarboxylate2-oxide, hereinafter, also known as 'PEA98')
[0120] In a 100ml round bottom flask, 4.69g of diisopropyl-L-tartrate (diisopropyl-L-tartrate, 20mmol) and 3.96g of pyridine (pyridine, 50mmol) were dissolved in 40ml of dichloromethane, after The temperature was lowered to 0 °C. Under a nitrogen atmosphere, 2.86 g of thionyl chloride (24 mmol) was slowly injected for 30 minutes, and then the temperature was raised to normal temperature. After reacting at normal temperature for 15 minutes, 30 ml of distilled water was added to complete the reaction. After separating the organic layer and the aqueous layer, the aqueous layer was extracted again with 20 ml of dichloromethane, thereby collecting the organic layer, and, for several layers, after washing once with 20 ml of 1N HCl solution, washed with 20 ml of saturated sodium b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com