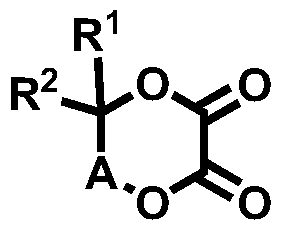
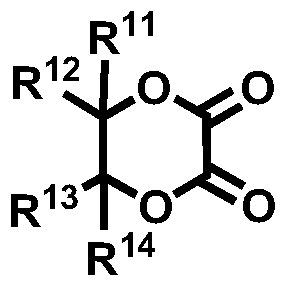
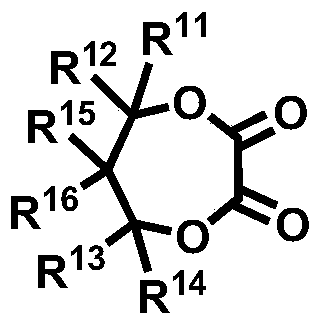
Novel compound and lithium secondary battery electrolyte containing it
A lithium secondary battery, secondary battery technology, applied in secondary batteries, lithium storage batteries, non-aqueous electrolyte storage batteries, etc., can solve the problems of high volatility and high flammability, and achieve improved expansion, higher discharge capacity, and higher capacity. The effect of recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] [Example 1] 5,5,6,6-tetramethyl-1,4-dioxane-2,3-dione (5,5,6,6-tetramethyl-1,4-dioxane-2, 3-dione, hereinafter, also referred to as 'PEA77') synthesis
[0144]
[0145] In a 100 ml round bottom flask, 2.36 g of pinacol (20 mmol) and 4.75 g of pyridine (60 mmol) were dissolved in 30 ml of tetrahydrofuran (THF), after which the temperature was lowered to 0°C. Under a nitrogen atmosphere, 3.05 g of oxalyl chloride (24 mmol) was slowly injected for 30 minutes, and then the temperature was raised to normal temperature. After reacting at normal temperature for 15 minutes, 30 ml of distilled water was added to complete the reaction. The organic layer was collected by two extractions with 20 ml of ethyl acetate, and, for several layers, washed once with 20 ml of 1N HCl solution, washed with 20 ml of saturated sodium bicarbonate (NaHCO 3 ) solution to wash once. Anhydrous magnesium sulfate (MgSO 4 ) after drying and concentrating the organic layer, the remaining solid was...
Embodiment 2
[0147] [Example 2] 6,6-dimethyl-[1,4]dioxepane-2,3-dione (6,6-dimethyl-[1,4]dioxepane-2,3-dione , hereinafter, also referred to as 'PEA79')
[0148]
[0149]In a 100ml round bottom flask, 2.08g of 2,2-dimethyl-1,3-propanediol (2,2-dimethyl-1,3-propanediol, 20mmol) and 4.75g of pyridine (pyridine, 60mmol) Dissolved in 30 ml of tetrahydrofuran (THF), after which the temperature was lowered to 0°C. Under a nitrogen atmosphere, 3.05 g of oxalyl chloride (24 mmol) was slowly injected for 30 minutes, and then the temperature was raised to normal temperature. After reacting at normal temperature for 15 minutes, 30 ml of distilled water was added to complete the reaction. The organic layer was collected by two extractions with 20 ml of ethyl acetate, and, for several layers, washed once with 20 ml of 1N HCl solution, washed with 20 ml of saturated sodium bicarbonate (NaHCO 3 ) solution to wash once. Anhydrous magnesium sulfate (MgSO 4 ) after drying and concentrating the organ...
Embodiment 3
[0151] [Example 3] 5,6-dioxy-1,4-dioxane-2,3-dicarboxylic acid (2R,3R)-diethyl ester ((2R,3R)-diethyl5,6-dioxo-1, 4-dioxane-2,3-dicarboxylate, hereinafter also referred to as 'PEA82') synthesis
[0152]
[0153] In a 100ml round bottom flask, 4.12g of diethyl-L-tartrate (diethyl-L-tartrate, 20mmol) and 4.75g of pyridine (pyridine, 60mmol) were dissolved in 30ml of tetrahydrofuran (THF), after The temperature was lowered to 0 °C. Under a nitrogen atmosphere, 3.05 g of oxalyl chloride (24 mmol) was slowly injected for 30 minutes, and then the temperature was raised to normal temperature. After reacting at normal temperature for 15 minutes, 30 ml of distilled water was added to complete the reaction. The organic layer was collected by extracting twice with 20 ml of ethyl acetate, and, after washing once with 20 ml of 1N HCl solution, the organic layer was washed once with 20 ml of saturated sodium bicarbonate (NaHCO 3 ) solution to wash once. Anhydrous magnesium sulfate (M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com