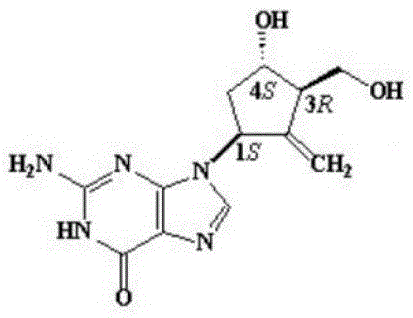
Entecavir-loaded mesoporous silica nanoparticle and preparation method thereof
A mesoporous silicon dioxide and silicon dioxide technology, applied in the field of medicine, can solve the problems of no intravenous controlled injection preparation method, can not be used for patients with emergency patients and cannot be administered orally, etc., to improve bioavailability, Improve the effect of drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1 mesoporous silica nanoparticles (MSNs)
[0024] Take 1.0g of cetyltrimethylammonium bromide, 0.28g of sodium hydroxide, add 480ml of distilled water, stir and dissolve in a water bath at 80°C, and after stabilizing for 0.5h, slowly add 2mL of ethyl orthosilicate dropwise, continue to stir for 2h, and then naturally cool down. Collect nanoparticles by centrifugation, add a mixed solution of methanol and concentrated hydrochloric acid (3ml, 37.4%), heat at reflux for 24h, collect nanoparticles by centrifugation, wash with water and methanol 7 times, and dry in vacuo to obtain MSNs.
[0025] The average particle size of MSNs measured by high-resolution electron microscope is 100nm, and the average pore size is 3.52nm.
Embodiment 2
[0027] Take entecavir 0.5g, add the mixed solution 4ml of DMSO and dichloromethane (DMSO0.8ml, dichloromethane 3.2ml) and make it dissolve, add the MSNs1.0g that embodiment 1 makes, ultrasonic 15min, disperse evenly, then in Stir at 25° C. for 24 h at a stirring speed of 100 rpm. The above solution was centrifuged at 12,000 rpm for 10 min, the supernatant was discarded, and the precipitate was retained. The precipitate was placed in a vacuum drying oven and dried in vacuum for 24 hours to obtain entecavir-loaded mesoporous silica nanoparticle powder.
[0028] According to the method of Example 4, the drug loading amount is 25.11%.
Embodiment 3
[0030] Weigh 0.5 g of Entecavir, add 4 ml of mixed solution of DMSO and ethanol (DMSO 1 ml, ethanol 3 ml) to dissolve it, add 1.0 g of MSNs prepared in Example 1, sonicate for 20 min, disperse evenly, then stir at 25 ° C for 20 h, stir The speed is 150rpm. The above solution was centrifuged at 12,000 rpm for 10 min, the supernatant was discarded, and the precipitate was retained. The precipitate was placed in a vacuum drying oven and dried in vacuum for 24 hours to obtain entecavir-loaded mesoporous silica nanoparticle powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com