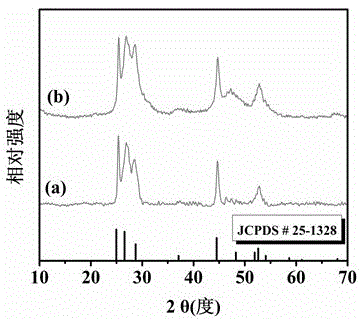
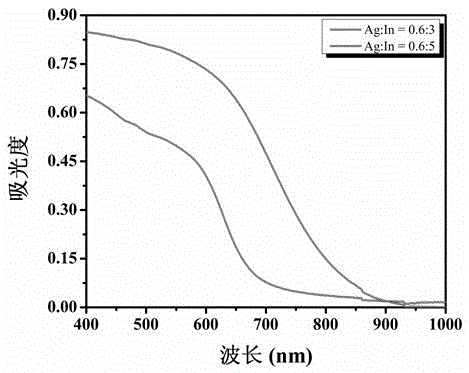
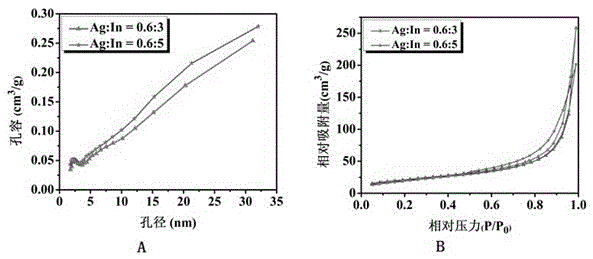
Method for preparing narrow-bandgap In-rich type AgInS2 photocatalyst with visible-light response
A photocatalyst and narrow bandgap technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problems of narrow band gap, complex operation process, harsh preparation conditions, etc., and achieve band gap The effect of narrow width, easy operation, and short preparation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Add 0.6mmolAgNO 3 and 3mmolIn(NO 3 ) 3 Dissolved in 170mL ethanol, ultrasonically dissolved to form a transparent solution of the metal salt precursor.
[0027] (2) Dissolve 10mmol thioacetamide in 10mL ethanol and stir vigorously to form a precipitant. Add the precipitating agent solution dropwise into the above metal salt precursor solution under continuous stirring.
[0028] (3) Transfer the mixed solution to a 250mL three-necked flask, add a condensing reflux device, and stir at an appropriate speed, and the temperature of the water bath is 55 o C, the water bath time is 3 hours.
[0029] (4) After the reaction, age for several hours, discard the yellow supernatant, centrifuge the reddish-brown precipitate, wash with deionized water and absolute ethanol three times, and then wash at 60-100 o Dry at C for a certain period of time, cool naturally, and grind to obtain a narrow bandgap indium-rich AgInS with a controllable and adjustable spectral absorption ran...
Embodiment 2
[0031] (1) Add 0.6mmolAgNO 3 and 3mmolIn(NO 3 ) 3 Dissolved in 170mL ethylene glycol, ultrasonically dissolved to form a transparent solution of the metal salt precursor.
[0032] (2) Dissolve 10mmol thioacetamide in 10mL ethylene glycol and stir vigorously to form a precipitant. Add the precipitating agent solution dropwise into the above metal salt precursor solution under continuous stirring.
[0033] (3) Transfer the mixed solution to a 250mL three-necked flask, add a condensing reflux device, and stir at an appropriate speed, and the temperature of the water bath is 70 o C, the water bath time is 3 hours.
[0034] (4) After the reaction, age for several hours, discard the yellow supernatant, centrifuge the reddish-brown precipitate, wash with deionized water and absolute ethanol three times, and then wash at 60-100 o Dry at C for a certain period of time, cool naturally, and grind to obtain a narrow bandgap indium-rich AgInS with a controllable and adjustable spectra...
Embodiment 3
[0036] (1) Add 0.6mmol CH 3 COOAg and 3mmolIn 2 (SO 4 ) 3 Dissolve in 170mL propanol, ultrasonically dissolve to form a transparent solution of the metal salt precursor.
[0037](2) Dissolve 5mmol thioacetamide in 10mL propanol and stir vigorously to form a precipitant. Add the precipitating agent solution dropwise into the above metal salt precursor solution under continuous stirring.
[0038] (3) Transfer the mixed solution to a 250mL three-necked flask, add a condensing reflux device, and stir at an appropriate speed, and the temperature of the water bath is 95 o C, the water bath time is 3 hours.
[0039] (4) After the reaction, age for several hours, discard the yellow supernatant, centrifuge the reddish-brown precipitate, wash three times with deionized water and absolute ethanol, and then wash at 60-100 o Dry at C for a certain period of time, cool naturally, and grind to obtain a narrow bandgap indium-rich AgInS with a controllable and adjustable spectral absorpt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com