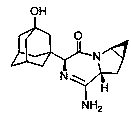
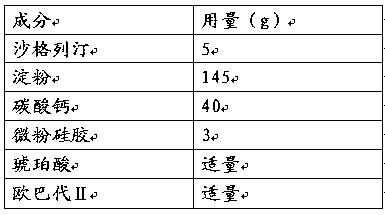
Saxagliptin medicinal preparation
A technology of pharmaceutical preparations and fillers, which is applied in the field of saxagliptin pharmaceutical preparations, can solve the problems of uniform dispersion, difficulty in controlling content uniformity, and inability to accurately control content uniformity, so as to reduce degradation, reduce types of excipients, and ensure drug effective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] Preparation:
[0038] (1) Mix microcrystalline cellulose and calcium hydrogen phosphate, add hydrochloric acid solution to granulate, dry, granulate, the measured particle size is 0.60mm; the particle pH is 4.2;
[0039] (2) Mix the above granules with magnesium stearate, micronized silica gel and saxagliptin evenly;
[0040] (3) Press into tablets, and coat with Opadry II.
Embodiment 2
[0042]
[0043] Preparation:
[0044] (1) Mix starch and calcium carbonate, add succinic acid solution to granulate, dry, granulate, the measured particle size is 0.64mm; the pH of the granules is 5.3;
[0045] (2) Mix the above granules with micronized silica gel and saxagliptin evenly;
[0046] (3) Press into tablets, and coat with Opadry II.
Embodiment 3
[0048]
[0049] Preparation:
[0050] (1) Mix microcrystalline cellulose and sucrose, add fumaric acid solution to granulate, dry, granulate, the measured particle size is 0.4mm; the particle pH is 4.8;
[0051] (2) Mix the above granules with magnesium stearate, talcum powder and saxagliptin evenly;
[0052] (3) Press into tablets, and coat with Opadry II.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com