Defluorinated canagliflozin compound, and preparation method and application thereof
A deflucagliflozin and reaction technology, applied in organic chemistry, measuring devices, instruments, etc., can solve problems affecting drug efficacy, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
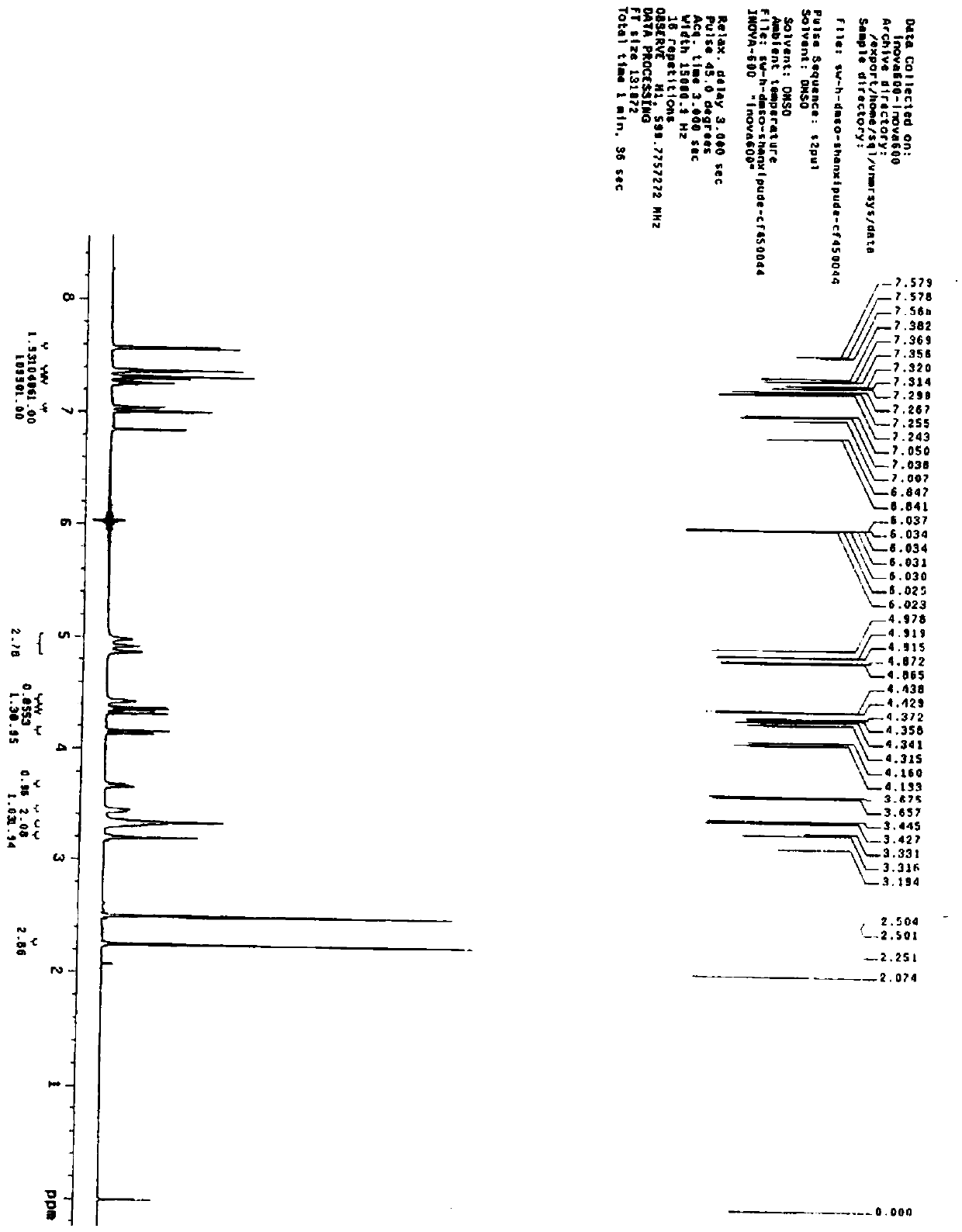
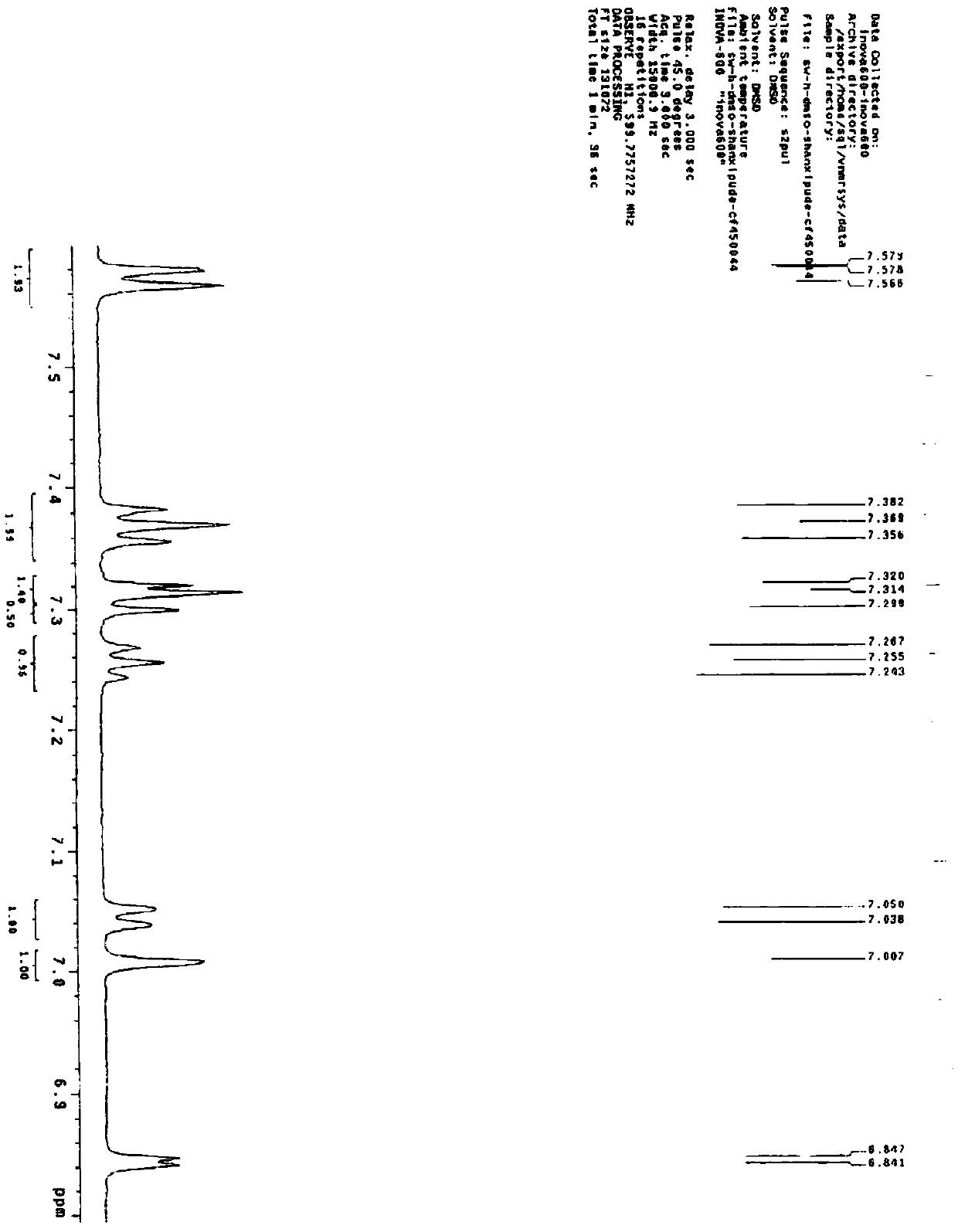
[0038] Preparation of desflucagliflozin compound
[0039]A: Add toluene (10L) into a 20L glass reactor, start stirring, and add 2-[(5-bromo-2-methylphenyl)methyl]-5-phenylthiophene and sodium iodide sequentially under nitrogen protection , Cuprous iodide. After stirring for 15 minutes, N,N-dimethylethylenediamine and diglyme were added, and the reaction solution was heated to 85-90° C. and stirred for 36 hours. Cool down to 45-50°C, add activated carbon, stir at the same temperature for 1 hour, cool down to 25-30°C and filter, add the filtrate into a 50L reaction kettle filled with ethyl acetate. Wash once with 5% ammonia water, separate the organic layer, wash once with purified water, separate the layers, dry the organic phase with anhydrous sodium sulfate for 2 hours, filter, and concentrate the filtrate under reduced pressure at 35-40°C. Add the concentrated solution into methanol, stir at 70-75°C for 2 hours, cool, wait until the temperature drops to 20-25°C, filter, wa...
Embodiment 2
[0054] Desflucagliflozin compound detection method
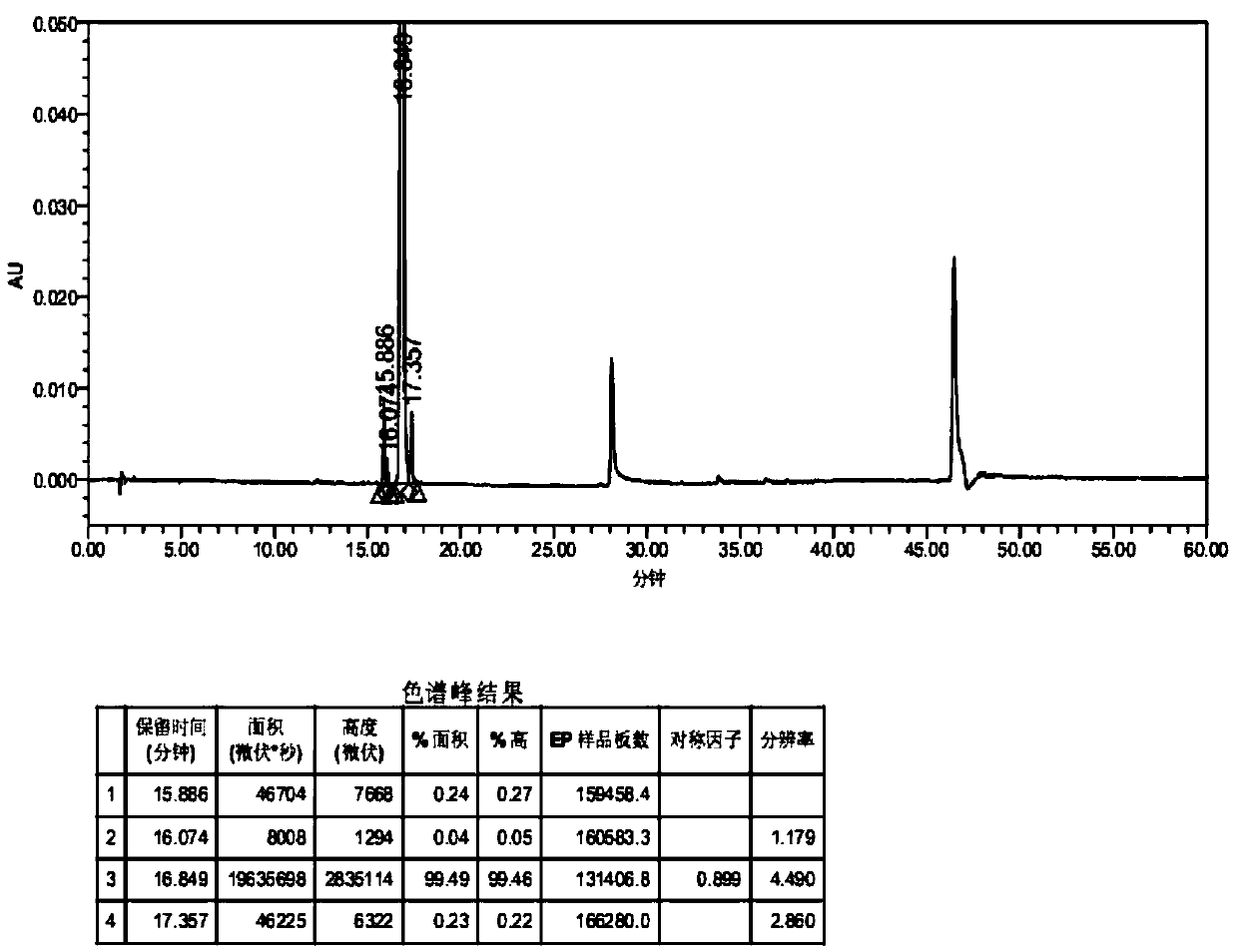
[0055] Chromatographic conditions: octadecylsilane bonded silica gel as filler, mobile phase A is acetonitrile-water (0.02% trifluoroacetic acid) (10:90), mobile phase B is acetonitrile-water (0.02% trifluoroacetic acid) (90︰10), the gradient elution is as follows:
[0056]
[0057] Column temperature: 45, °C flow rate: 1.0ml / min, injection volume: 20μl, detection wavelength: 290nm. The number of theoretical plates is not less than 2000 based on the net calculation of canagliflozin.
[0058] Assay method: accurately weigh the appropriate amount of composition fine powder (equivalent to canagliflozin C 24 h 25 FO 5 S. 1 / 2 h 2 (2.12.5mg), in the 25ml measuring bottle, add 0.025mg / ml desflecagliflozin stock solution 1ml, add solvent and be diluted to scale, shake up, filter, get continued filtrate as need testing solution. Precisely measure 20 μl of the test solution, inject it into the liquid chromatograph, and re...
Embodiment 3
[0060] Desflucagliflozin as a reference substance for the determination of related substances in canagliflozin raw materials
[0061] Measure according to high performance liquid chromatography (Chinese Pharmacopoeia 2010 edition two appendix Ⅴ D), chromatographic conditions with reference to embodiment 2.
[0062] Preparation of the test solution Weigh about 12.5mg of this product, weigh it accurately, put it in a 25ml measuring bottle, dissolve it with acetonitrile-water (50:50) and dilute to the mark, shake well, and you get it.
[0063] Preparation of desflucagliflozin reference substance solution Take an appropriate amount of desflucagliflozin reference substance, accurately weighed, dissolve and dilute into a 0.75 μg / ml solution with acetonitrile-water (50:50), as desflucagliflozin Gliflozin reference substance solution.
[0064] Determination method Precisely measure 20 μl of the test solution and the control solution, inject them into the liquid chromatograph, and rec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com