Application of cucurbitacine in preparing TrkA kinase inhibitor medicines
A technology of cucurbitacin and its use, which is applied in the field of cucurbitacin in the preparation of TrkA kinase inhibitor drugs, and can solve the problems that cucurbitacin inhibits TrkA kinase prevention, alleviation and treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Inhibitory Activity of Cucurbitacin B on Tyrosine Kinase and Serine / Threonine Kinase
[0045] Cucurbitacin B was dissolved in DMSO to obtain a 10 mM stock solution, which was then diluted with DMSO to obtain a 25.5 μM solution and further diluted with assay buffer to obtain a concentration of 0.5 μM. Cucurbitacin B was tested at a concentration of 500 nM. The high-throughput assays for the inhibitory activities of tyrosine kinases and serine / threonine kinases are shown in Table 1.
[0046] Table 1 High-throughput test for the inhibitory activity of cucurbitacin B on protein kinases
[0047]
[0048]
[0049] The results show that cucurbitacin B can inhibit TrkA activity at a concentration of 500nM to only 5% residual activity (*residual activity is relative to the protein kinase activity of 100%, how much activity is left), this test directly proves that cucurbitacin B has A new target - TrkA.
Embodiment 2
[0050] Embodiment 2 The effect of the compounds of the present invention on the activity of protein kinases in cell models
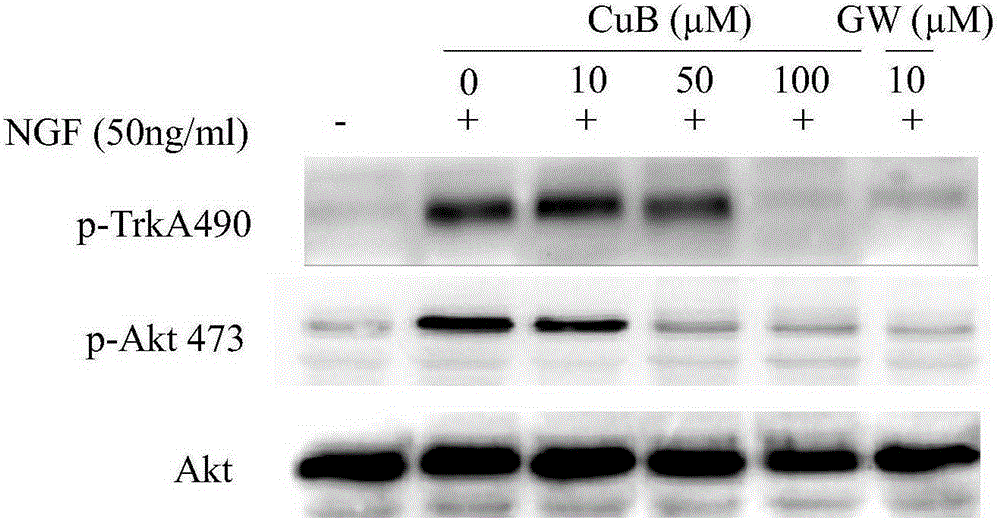
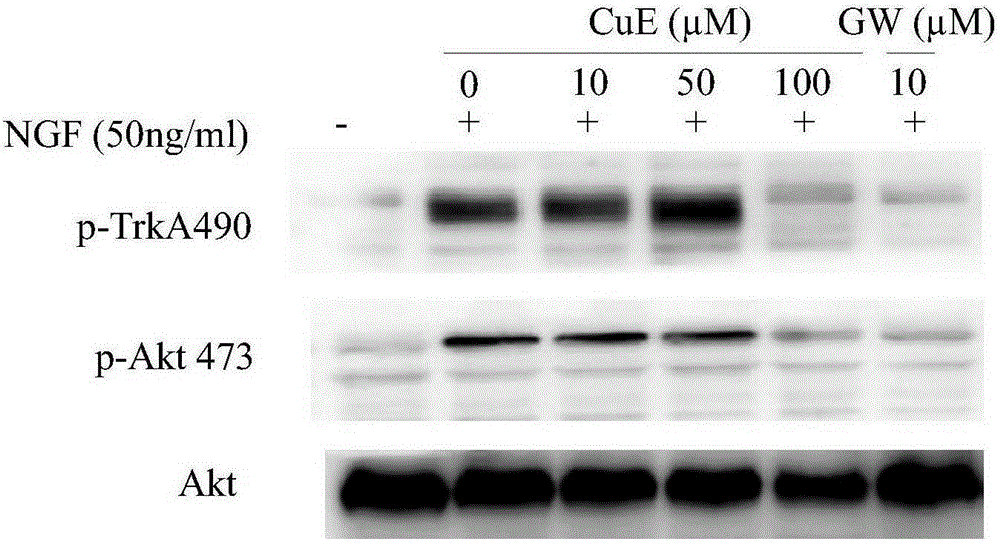
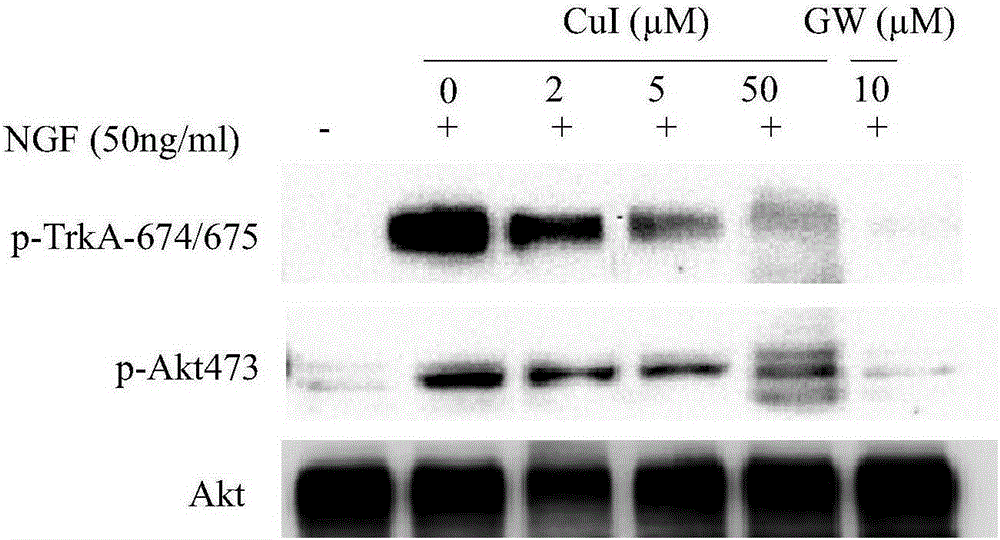
[0051] PC-12 cells according to 1 x 10 7 cells / well in a flat-bottomed 6-well plate, and add cucurbitacin B, cucurbitacin E, and cucurbitacin I. After 6 hours of serum starvation culture, NGF was added with a final concentration of 50ng / ml and incubated for 10min. Collect the cells, centrifuge at 600G for 5 minutes to remove the supernatant, wash once with PBS, take the cell pellet, and add cell lysate to lyse the cells. The protein was collected for Western-blot detection. The kinases to be tested were phosphorylated TrkA490 site, TrkA674 / 675 site and phosphorylated Akt473 site.
[0052] The experimental results are as Figure 1-3 shown.
[0053] figure 1 The results show that cucurbitacin B, like the positive control TrkA phosphorylation inhibitor-GW441756, can inhibit protein kinase TrkA490 site and Akt473 site (signal transduction and transcript...
Embodiment 3
[0062] The therapeutic effect of embodiment 3 cucurbitacin B and cucurbitacin E on mouse skin psoriasis
[0063] Preparation of experimental animals:
[0064] BALB / c mice were anesthetized by intraperitoneal injection of pentobarbital sodium (80mg / kg), and their back hair was shaved to form an exposed area with a size of about 2cm×3cm. They were reared in single cages for 1 day and then treated with drugs according to groups. .
[0065] Experimental grouping and administration:
[0066] All the mice were randomly divided into a normal control group (control), a model group (model), a dexamethasone (Dex) group and an experimental group. 6 in each group. Control group (control): smear appropriate amount of Vaseline on the bare back of mice every day, and at the same time gavage with normal saline, 0.4mL / (time / d); model group (Model): smear 5% Vaseline on the bare back of mice regularly every day Mizamod cream 42mg, at the same time with normal saline gavage, 0.4mL / (time / d); ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com