Method for preparing cyclopentanol
A technology of cyclopentanol and cyclopentene, which is applied in hydroxyl addition preparation, organic chemistry and other directions, can solve the problems of increasing post-processing energy consumption, increasing system viscosity, and difficulty in separation, reducing energy consumption and side reactions. , the effect of increasing the conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
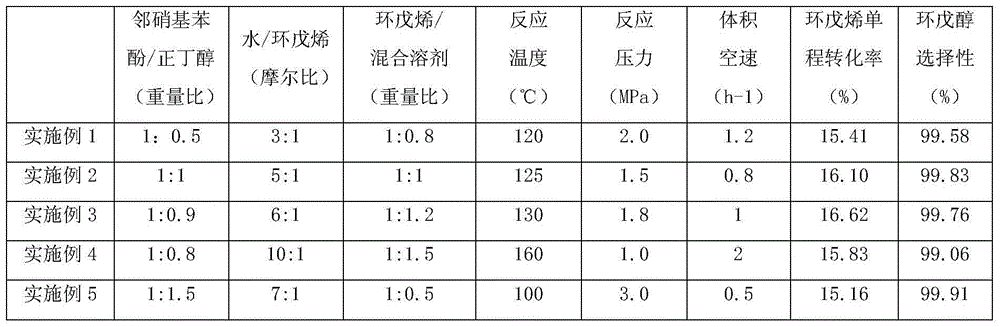
[0018] The material composed of cyclopentene, water, and mixed solvents is continuously passed through a fixed bed filled with a catalyst for hydration reaction. The catalyst is a strongly acidic cation exchange resin (purchased from Tianjin Weide International Trade Co., Ltd. Amberlyst35), the reaction temperature is 100-160°C, the reaction pressure is 1.0-3.0MPa, and the volume space velocity is 0.5-2hr -1 , wherein: the mixed solvent is composed of o-nitrophenol and n-butyl ketone with a weight ratio of (0.5~1.5):1, the weight ratio of the mixed solvent and cyclopentene is (0.5~1.5):1, water and cyclopentene and The molar ratio is 1:(3.0~10.0); after the reaction, the reactants are cooled and stratified into oil phase and water phase, the oil phase material is rectified under normal pressure, and the fraction at 38~55°C is condensed to obtain unreacted cyclopentene , 135 ~ 165 ° C distillate condensation collected refined cyclopentanol, the bottom liquid is rich in o-nitrop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com