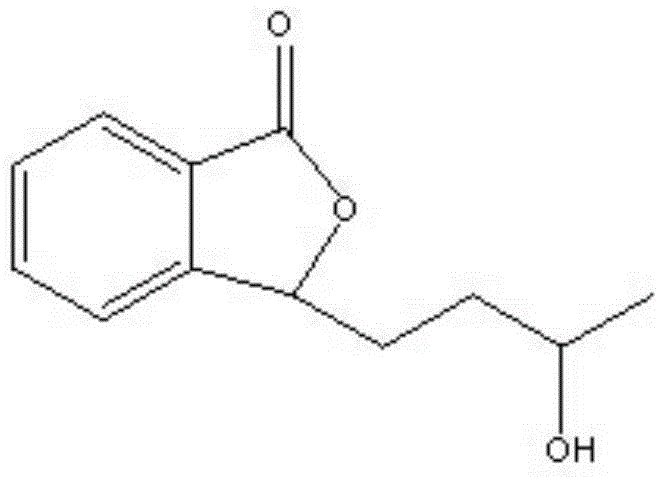
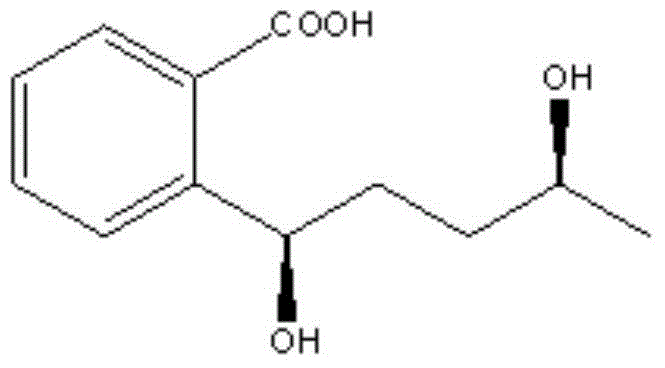
Novel compounds and applications thereof
A compound, pentanediol technology, applied in the field of new compounds, can solve the problem of no pharmacological activity, and achieve the effects of excellent cardiovascular and cerebrovascular pharmacological activity, improvement of neurological symptoms, and inhibition of thrombosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Effects on neurological symptoms and cerebral infarction range in middle cerebral artery thrombosis model (Middle Cerebral Artery Thrombosis, MCAT) rats.
[0060] 1. Animals
[0061]SD rats, half male and half male, weighing 190-250 g.
[0062] 2. Drugs and reagents
[0063] Test drug
[0064] 2-(1,4-pentanediol)benzoic acid, 2-(1,4-pentanediol)benzoic acid N,N'-dibenzylethylenediamine salt, 2-(1S,4S-pentanediol ) Benzoic acid, 2-(1S, 4S-pentanediol) benzoic acid N, N'-dibenzylethylenediamine salt, 2-(1S, 4R-pentanediol) benzoic acid, 2-(1S, 4R -pentanediol) benzoic acid N,N'-dibenzylethylenediamine salt.
[0065] Reagent
[0066] FeCl3*6H20 (A.R.), prepared with 1mol / L hydrochloric acid; red tetrazolium (TTC), (C.P.).
[0067] 3. Instruments
[0068] XTT solid microscope; constant temperature water bath oscillator; electronic analytical balance.
[0069] 4. Test methods and results
[0070] (1) Effects on neurological symptoms of MCAT rats
[0071]...
Embodiment 2
[0092] Example 2: Effects on Thrombosis of Arterial-Venous Bypass in Rats This example selects:
[0093] 1. Animals
[0094] SD rats, half male and half male, weighing 220-260 g.
[0095] 2. Drugs and reagents
[0096] Test drug
[0097] 2-(1,4-pentanediol)benzoic acid, 2-(1,4-pentanediol)benzoic acid N,N'-dibenzylethylenediamine salt, 2-(1S,4S-pentanediol ) Benzoic acid, 2-(1S, 4S-pentanediol) benzoic acid N, N'-dibenzylethylenediamine salt, 2-(1S, 4R-pentanediol) benzoic acid, 2-(1S, 4R -pentanediol) benzoic acid N,N'-dibenzylethylenediamine salt.
[0098] 3. Instruments
[0099] AEG-220 electronic analytical balance; Df-206 blast drying oven.
[0100] 4. Test methods and results
[0101] 1. See Table 4 for grouping
[0102]
[0103]
[0104] Table 4
[0105] 2. Administration
[0106] Oral administration was administered once a day for 3 consecutive days, and the thrombosis model group was given the same amount of vehicle. Surgery was performed after the la...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com