A novel degradable bone implant and its preparation method
A bone implant, a new type of technology, used in medical science, prosthesis, tissue regeneration, etc., can solve the problems of slow degradation rate and acidic degradation products, reduce toxicity, improve biocompatibility, and reduce freeness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
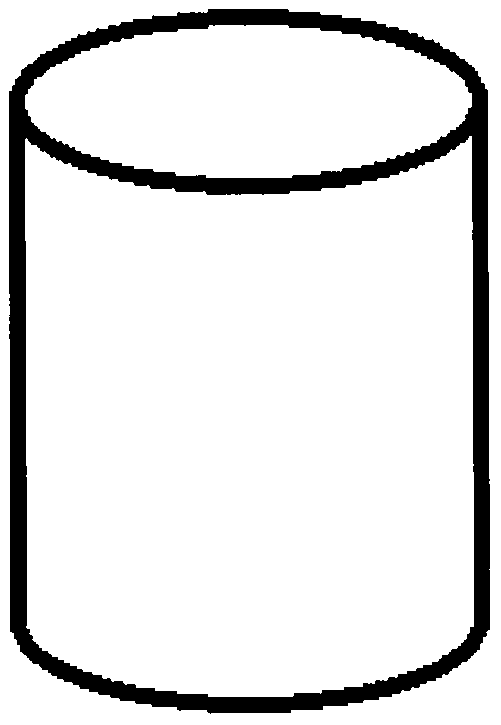
[0064] figure 1 It is a schematic diagram of the degradable bone filling particles prepared by the present invention, and the specific preparation steps are as follows:
[0065] a. According to the formula ①, the powder and the liquid are mixed and stirred to obtain a paste;
[0066] b. Inject the paste into a φ3x3 mold and keep the injection pressure at 8atm;
[0067] c. Demoulding after the paste is cured to obtain bone filling particles;
[0068] d. Place the bone filling particles in a constant temperature and humidity cabinet with a temperature of 37° C. and a humidity of 60% for 28 hours.
[0069] The prepared degradable bone filling particles can be used for bone defect filling.
Embodiment 2
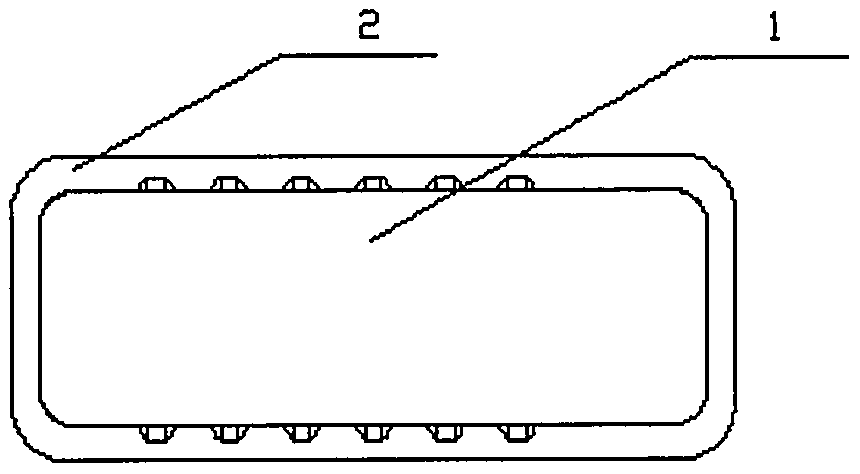
[0071] figure 2 The structural schematic diagram of the degradable fusion device 1 of the metal frame 2 containing magnesium alloy prepared for the present invention, the specific preparation steps are as follows:
[0072] a. Process the magnesium alloy into a frame and put it into a mold.
[0073] b. According to the formula ②, the powder and the liquid are mixed and stirred to obtain a paste;
[0074] c. Inject the paste into a mold with a magnesium alloy frame and keep the injection pressure at 15atm;
[0075] d. Release the mold after the paste solidifies to obtain a biodegradable fuser;
[0076] e. Place the biodegradable fuser in a constant temperature and humidity cabinet with a temperature of 37° C. and a humidity of 80% for 48 hours.
[0077] The prepared degradable fusion device can be used in various fusion operations.
Embodiment 3
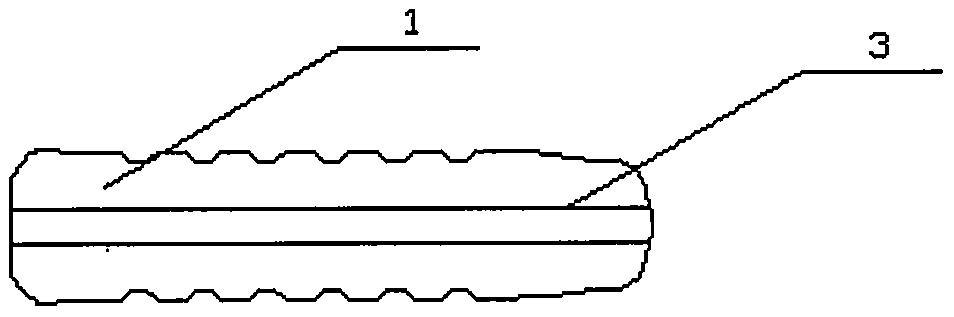
[0079] image 3 The structural schematic diagram of the degradable fusion device 1 containing the metal inner support 3 of the pure magnesium strip prepared for the present invention, the specific preparation steps are as follows:
[0080] a. Process pure magnesium into strips and put them into molds.
[0081] b. According to the formula ③, the powder and the liquid are mixed and stirred to obtain a paste;
[0082] c. Inject the paste into a mold with a pure magnesium rack, and keep the injection pressure at 12atm;
[0083] d. Release the mold after the paste solidifies to obtain a biodegradable fuser;
[0084] e. Place the biodegradable fuser in a constant temperature and humidity cabinet with a temperature of 37° C. and a humidity of 90% for 36 hours.
[0085] The prepared degradable fusion device can be used in various fusion operations.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com