Biphenyl compound and application thereof
A compound, biphenyl technology, applied in application, organic chemistry, animal repellent, etc., can solve problems such as unreported compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1: the preparation of compound 1
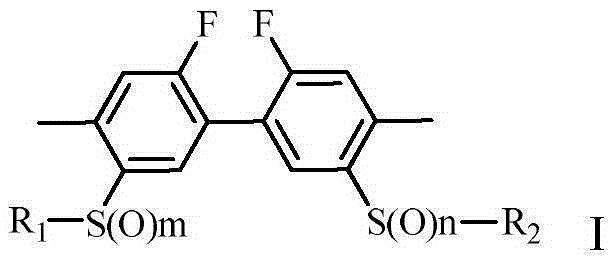
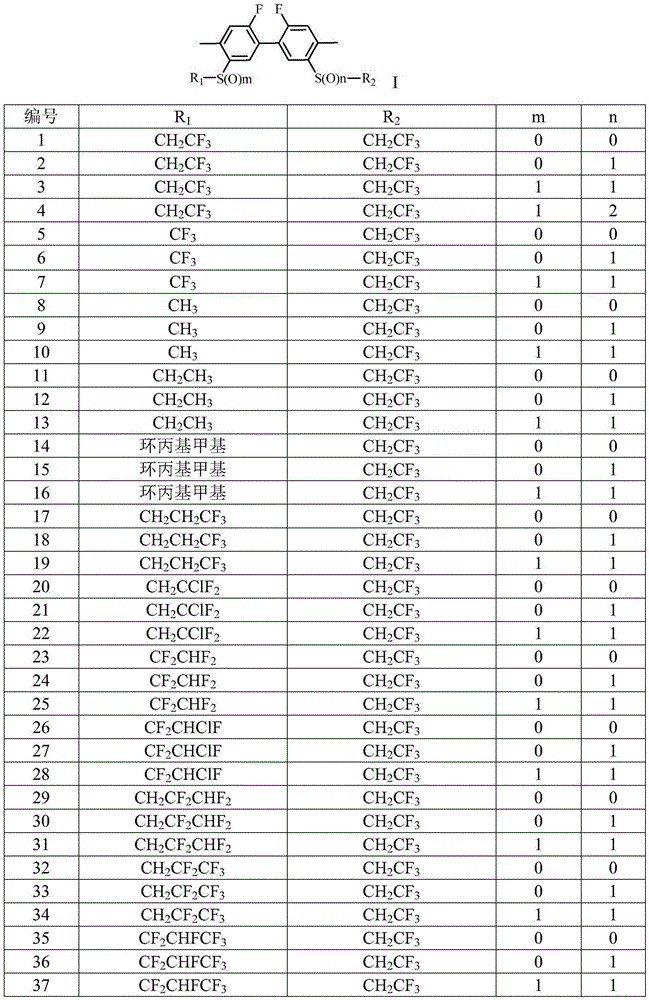
[0072]
[0073] 1.1, the preparation of intermediate IV-1
[0074] Weigh 3-(2,2,2-trifluoroethylthio)-4-methyl-6-fluoroaniline (intermediate III-1, which can be prepared by referring to methods reported in WO2010100189, US2012053052, JP2012519662, EP2403837 or CN102341376, etc. (obtained) 10.00 g (41.80 mmol) was placed in a 500 ml three-necked flask, 60 ml of concentrated hydrochloric acid was added, the temperature was lowered to 0-5° C., and the reaction was stirred for 30 minutes. A 100 ml aqueous solution of 3.46 g (50.15 mmol) of sodium nitrite was slowly added dropwise therein, and during this process, the reaction temperature was maintained not to exceed 0-5°C. After the dropwise addition was completed, the stirring reaction was continued for 1 hour.
[0075] A 100 ml aqueous solution of 13.88 g (83.61 mmol) of potassium iodide was slowly added dropwise to the diazonium salt solution, and during this process, th...
Embodiment 2
[0080] Embodiment 2: the preparation of compound 2 and 3
[0081]
[0082] 2.00 g (4.48 mmol) of compound 1 was dissolved in 20 ml of chloroform, and the temperature was lowered to 0-5°C. At this temperature, 0.98 g (4.68 mmol, 85% purity) of m-chloroperoxybenzoic acid was added in three portions. The reaction mixture was stirred at a temperature of 0-5°C for 2 hours. After the reaction was monitored by TLC, the reaction solution was washed successively with aqueous sodium thiosulfate and aqueous sodium bicarbonate and dried with anhydrous magnesium sulfate, filtered, and precipitated under reduced pressure. The residue was column chromatographed (eluent was ethyl acetate and petroleum ether, the volume ratio is 1:6 to 1:3) to obtain 1.21 g of compound 2 (white solid) and 0.70 g of compound 3 (white solid) after purification. NMR and mass spectrometry data are as follows:
[0083] Compound 2: 1 HNMR (300MHz, internal standard TMS, solvent CDCl 3 )δ(ppm):2.45(s,3H),2.54...
Embodiment 3
[0087] Embodiment 3: Determination of acaricidal activity
[0088] The compound of the present invention was used to test the activity of Tetranychus cinnabarinus. The method of determination is as follows:
[0089] After the compound to be tested prepared in the above synthesis example was dissolved in acetone, it was diluted to the required concentration with water containing 0.1% Tween 80, and the content of acetone in the total solution was not more than 10%.
[0090] Taking Tetranychus cinnabarinus Boisduval as the target, the Airbrush spray method was used to measure the activity, and the pressure of the Airbrush spray treatment was 0.7kg / cm 2 , the spray volume is 0.5ml, and placed in the standard observation room after treatment. After 72 hours, the number of surviving mites was investigated and the mortality rate was calculated.
[0091] The test results are as follows:
[0092] When the concentration of the drug solution was 100mg / L, the lethality of compounds 1, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com