Polylysine nano prodrug micelle with charge turning and targeting functions and preparation and application thereof
A polylysine and charge inversion technology is applied in the application field of preparing tumor-targeted drugs to achieve the effects of increasing the ability to enter cells, avoiding protein adsorption, and avoiding metabolic burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
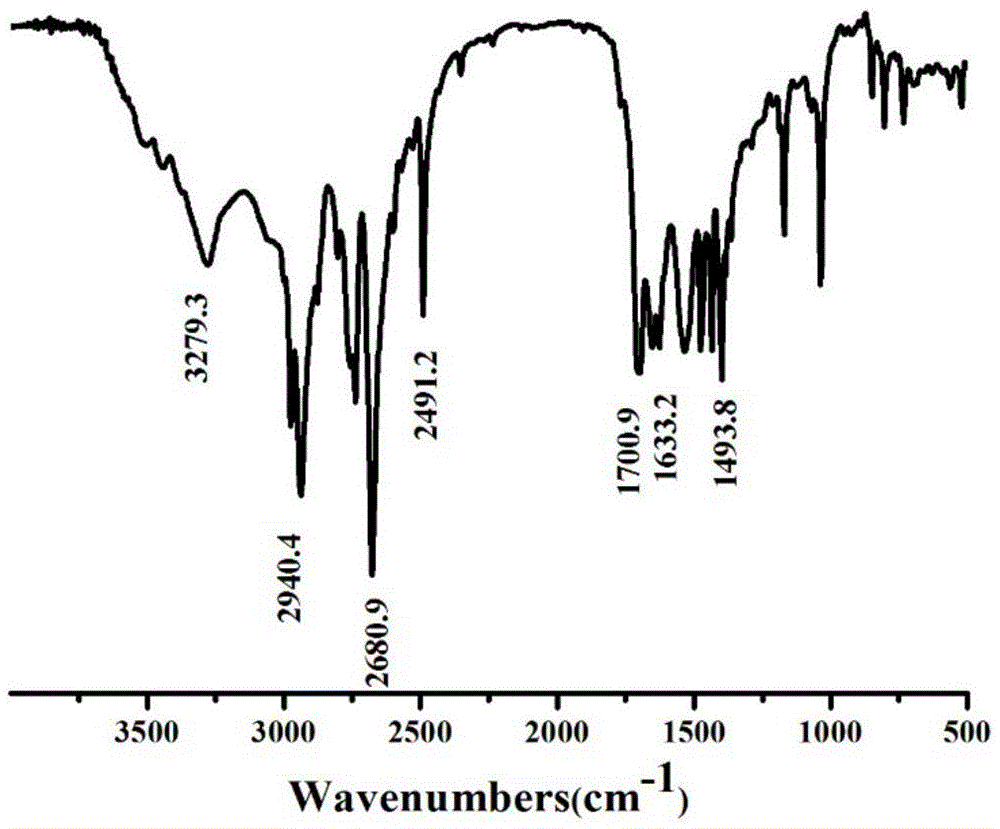
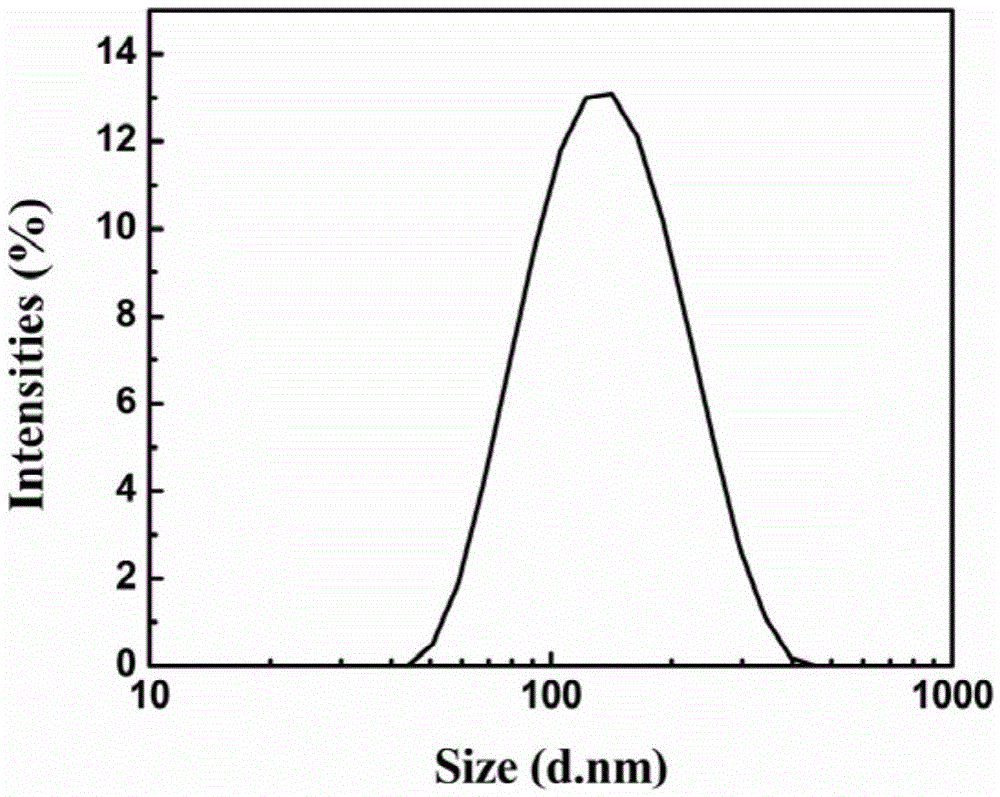
Image
Examples
Embodiment 1
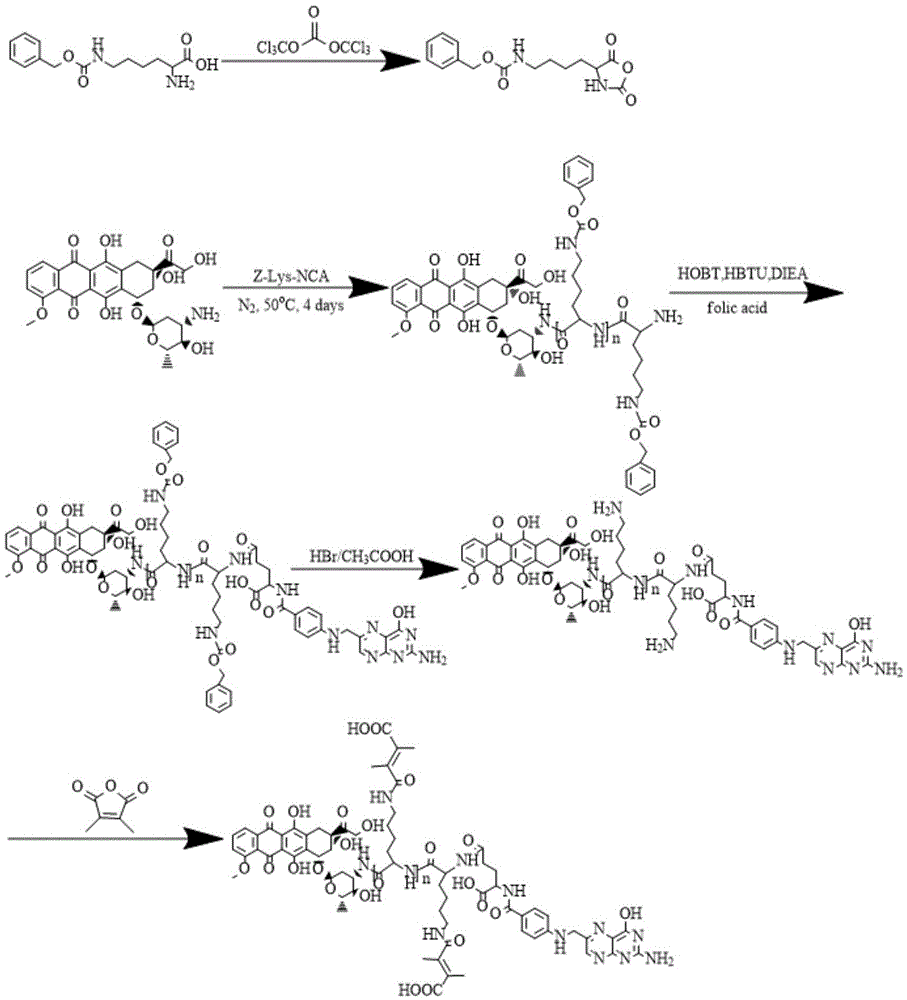
[0039] (1) Preparation of Nε-benzyloxycarbonyl-L-lysine anhydride by phosgene method: ultrasonically disperse 2.62 g (0.02 mol) of Nε-benzyloxycarbonyl-L-lysine in 90 mL of anhydrous THF. N 2Protected, vigorously stirred at 50°C. Dissolve 5.94g of recrystallized triphosgene in 15mL of anhydrous THF, and add it dropwise to the reaction system until the solution becomes clear, then continue stirring for 90min, and finally distill off about 2 / 3 of the triphosgene under reduced pressure. The solvent was then added into a large amount of n-hexane, and the solid was obtained by suction filtration, and then recrystallized twice, and the product was vacuum-dried at room temperature overnight to prepare Z-Lys-NCA.
[0040] (2) DOX-triggered NCA ring-opening polymerization to prepare DOX-PLL (Z): 20 mg of DOX was dissolved in 3 mL of anhydrous DMF, and 320 mg of Z-Lys-NCA prepared above was dissolved in 1 mL of anhydrous DMF. Slowly added dropwise to DOX / DMF solution, under N 2 Under...
Embodiment 2
[0056] (1) Preparation of Nε-benzyloxycarbonyl-L-lysine anhydride by phosgene method: ultrasonically disperse 2.62 g (0.02 mol) of Nε-benzyloxycarbonyl-L-lysine in 90 mL of anhydrous THF. N 2 Protected, vigorously stirred at 50°C. Dissolve 5.94g of recrystallized triphosgene in 15mL of anhydrous THF, and add it dropwise to the reaction system until the solution becomes clear, then continue stirring for 90min, and finally distill off about 2 / 3 of the triphosgene under reduced pressure. The solvent was then added into a large amount of n-hexane, and the solid was obtained by suction filtration, and then recrystallized twice, and the product was vacuum-dried at room temperature overnight to prepare Z-Lys-NCA.
[0057] (2) DOX-triggered NCA ring-opening polymerization to prepare DOX-PLL (Z): 20 mg of DOX was dissolved in 2.5 mL of anhydrous DMF, and 210 mg of Z-Lys-NCA prepared above was dissolved in 0.7 mL of anhydrous DMF. It was slowly added dropwise to the DOX / DMF solution u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydrated particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com