Progesterone membrane agent and its preparing method
A ketone film and progesterone technology, which is applied to the progesterone film for external vaginal use and the field of preparation thereof, can solve the problems of difficulty in ensuring accurate drug dosage, inability to stand up immediately, and a large proportion of suppository bases, thereby avoiding metabolic burden, High progesterone concentration, rapid melt release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 Preparation of progesterone film of the present invention
[0035] 1. Raw materials: progesterone 50g, polyvinyl alcohol 165g, sodium lauryl sulfate 8.75g, ethylparaben 0.25g, water 660ml;
[0036] 2. Preparation method:
[0037] Preparation of liquid A: Weigh 165g of PVA, slowly pour it into a beaker filled with 560ml of water, stir while adding, let it stand still, after fully swelling, heat in a water bath to completely dissolve the PVA, add 5% ethylparaben ethanol Solution 5ml, mix well.
[0038] Configuration of liquid B: Weigh 50 g of the progesterone bulk drug (passed through a No. 9 sieve), add it to 100 ml of 8.75% sodium lauryl sulfate solution, and stir.
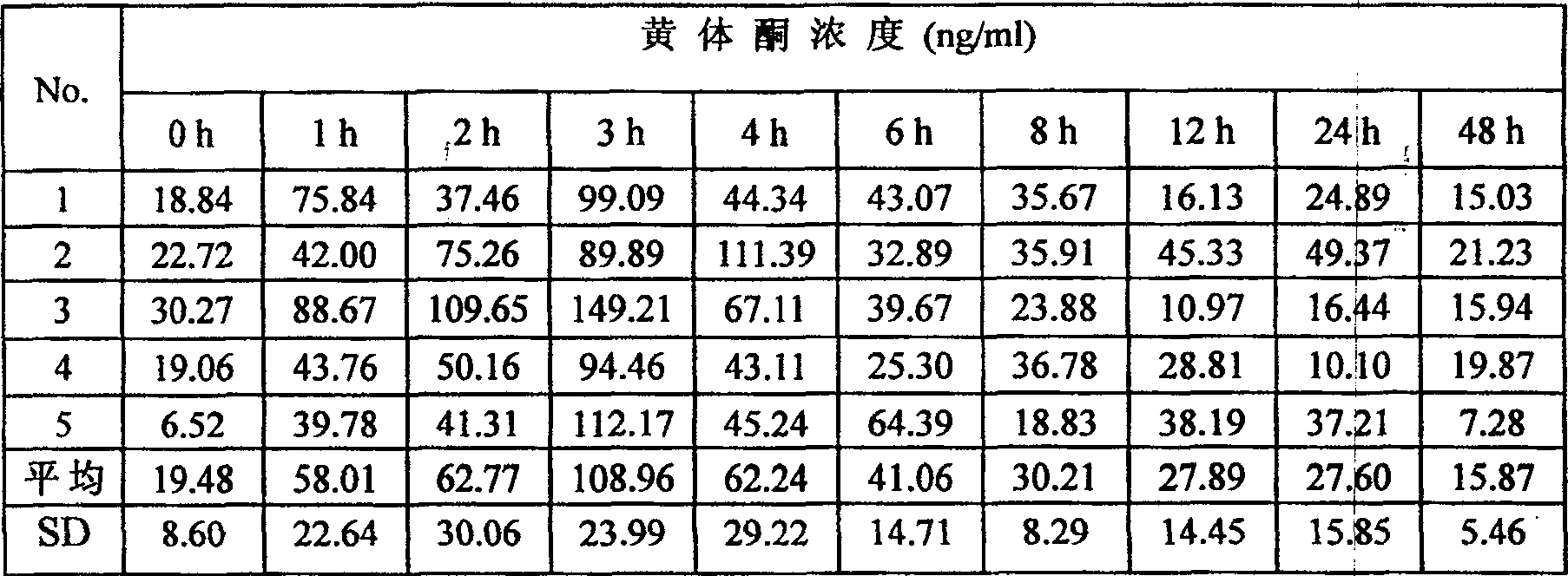
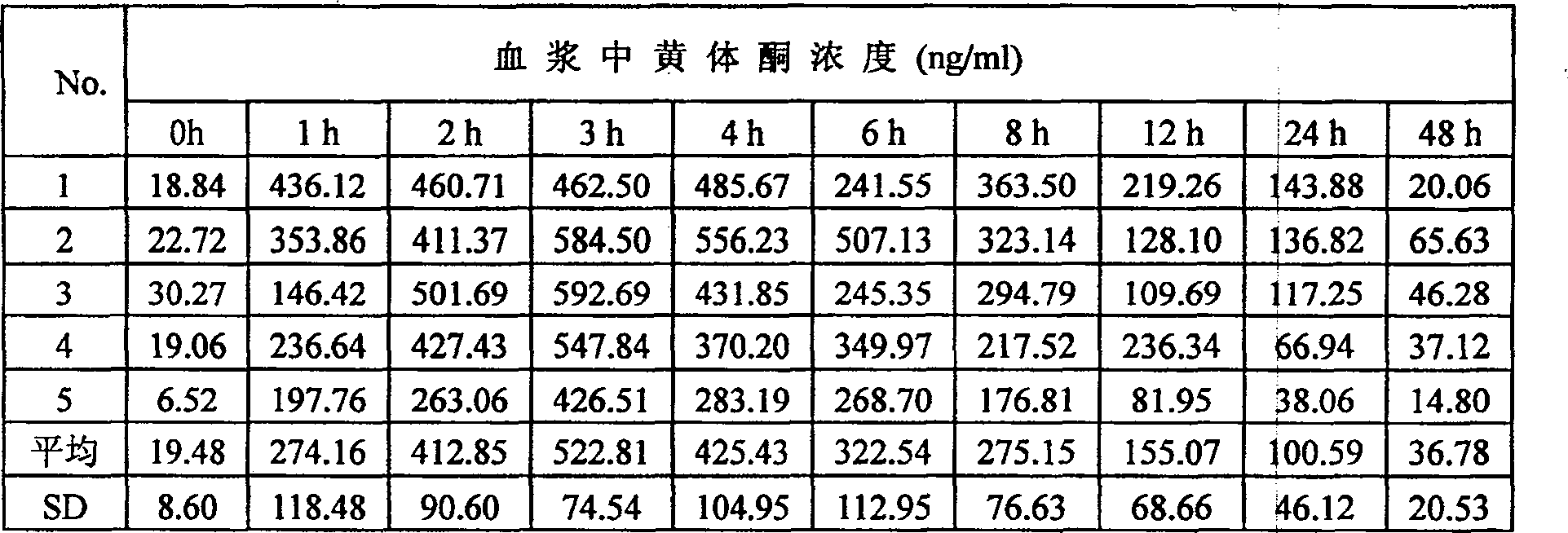
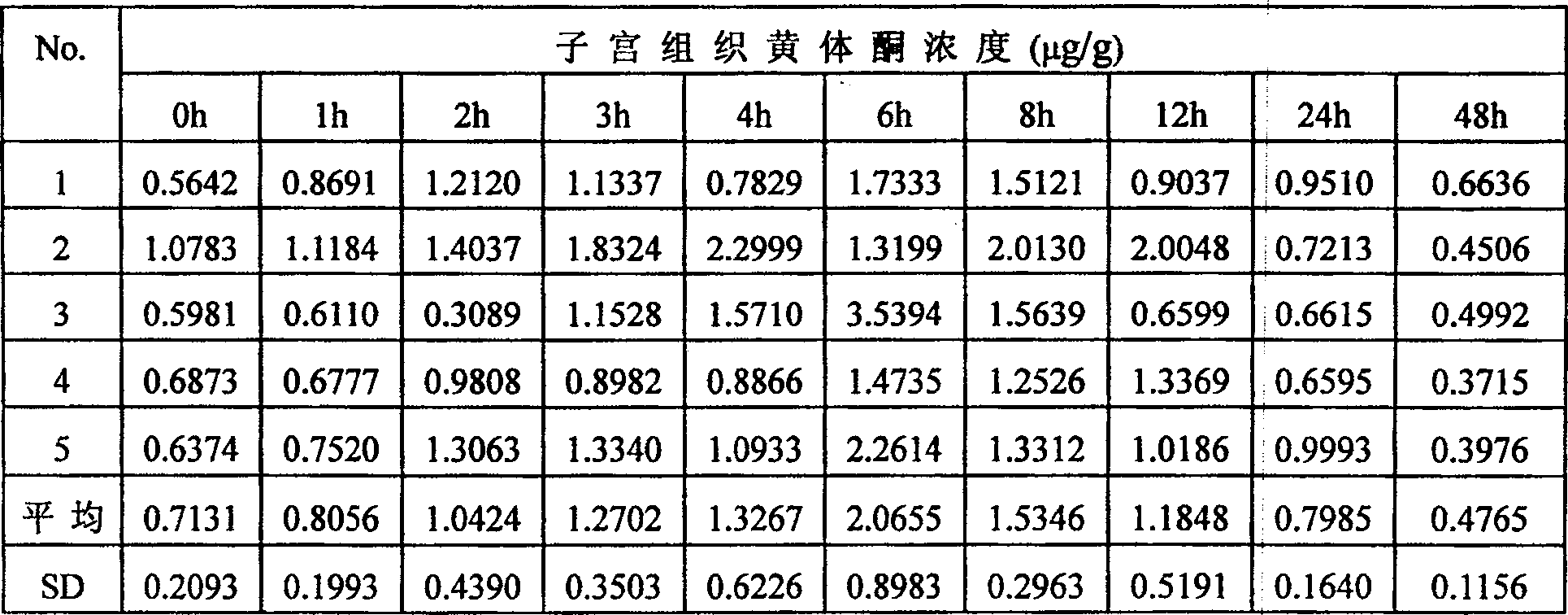
[0039] Pour liquid A into liquid B under stirring, stir for 15 minutes, and keep warm in a water bath at 60°C for 1 hour to remove air bubbles. Apply evenly, dry at 80°C until the surface of the film is not sticky, peel off the film, shape it, measure the drug content in the film per unit ar...
Embodiment 2
[0040] Embodiment 2 Preparation of progesterone film of the present invention
[0041] 1. Raw materials: progesterone 50g, polyvinyl alcohol 165g, sodium lauryl sulfate 8.75g;
[0042] 2. Preparation method:
[0043] Preparation of liquid A: Weigh 165g of PVA, slowly pour it into a beaker filled with 560ml of water, stir while adding, let it stand still, after fully swelling, heat in a water bath to completely dissolve the PVA;
[0044] Configuration of liquid B: Weigh 50 g of the progesterone bulk drug (passed through a No. 9 sieve), add it to 100 ml of 8.75% sodium lauryl sulfate solution, and stir.
[0045] Pour liquid A into liquid B under stirring, stir for 15 minutes, and keep warm in a water bath at 60°C for 1 hour to remove air bubbles. Apply evenly, dry at 80°C until the surface of the film is not sticky, peel off the film, shape it, measure the drug content in the film per unit area by high performance liquid chromatography, divide and pack according to the drug cont...
Embodiment 3
[0046] Embodiment 3 Preparation of progesterone film of the present invention
[0047] Raw materials: progesterone 75g, polyvinyl alcohol 245g, sodium lauryl sulfate 13.15g, ethylparaben 0.37g, water 990ml;
[0048] Progesterone film was prepared according to the method described in Example 1, so that its specification was 75 mg / tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com