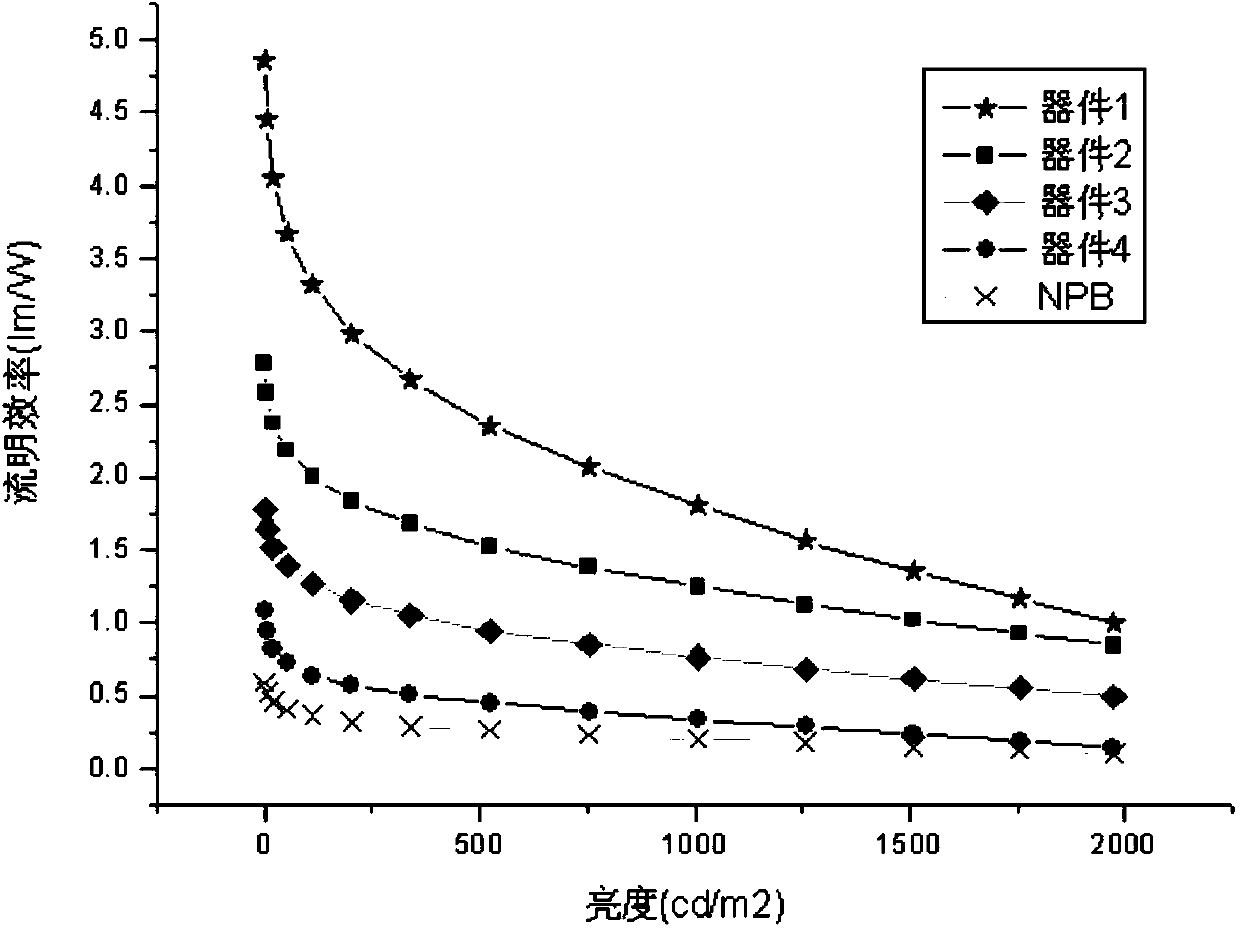
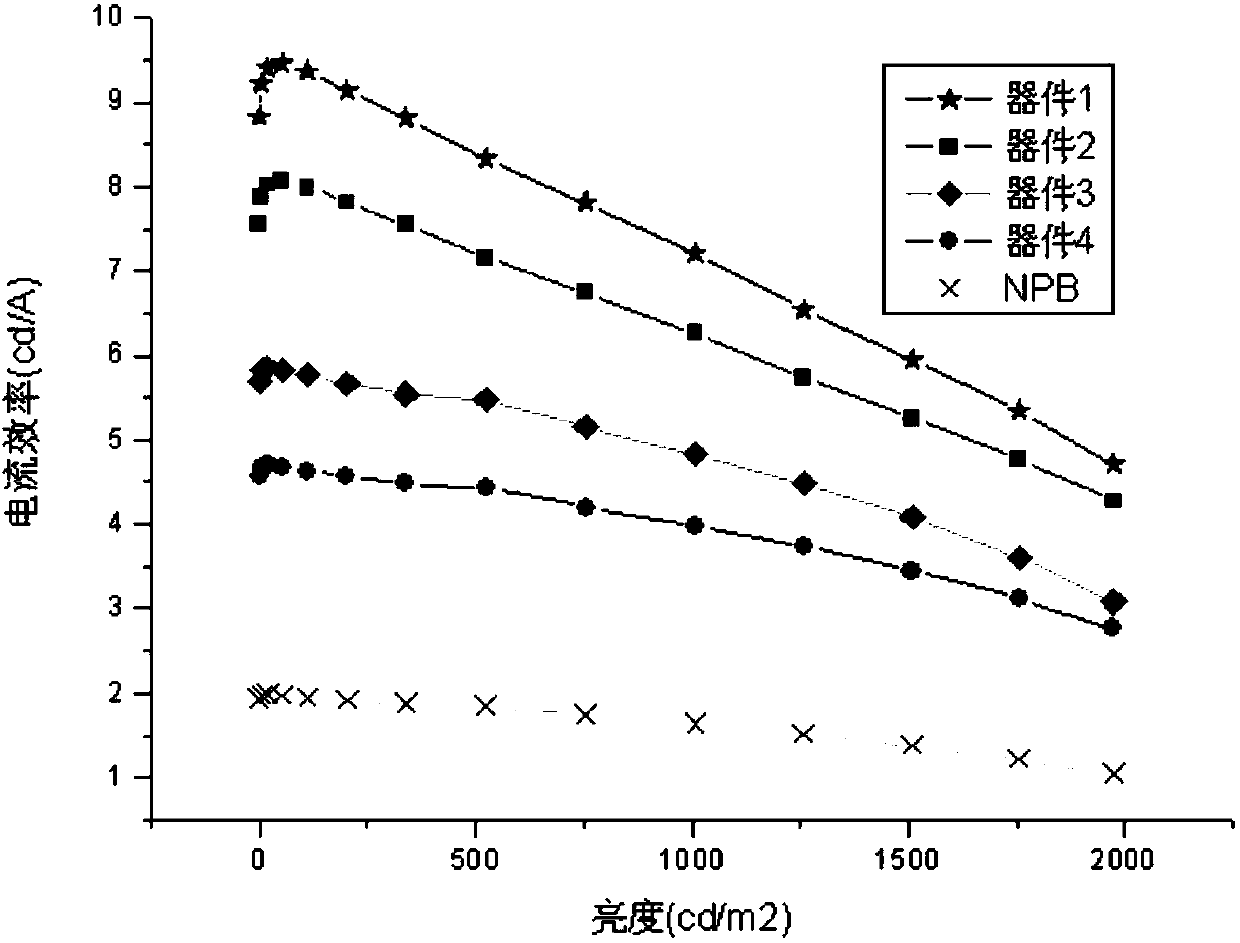
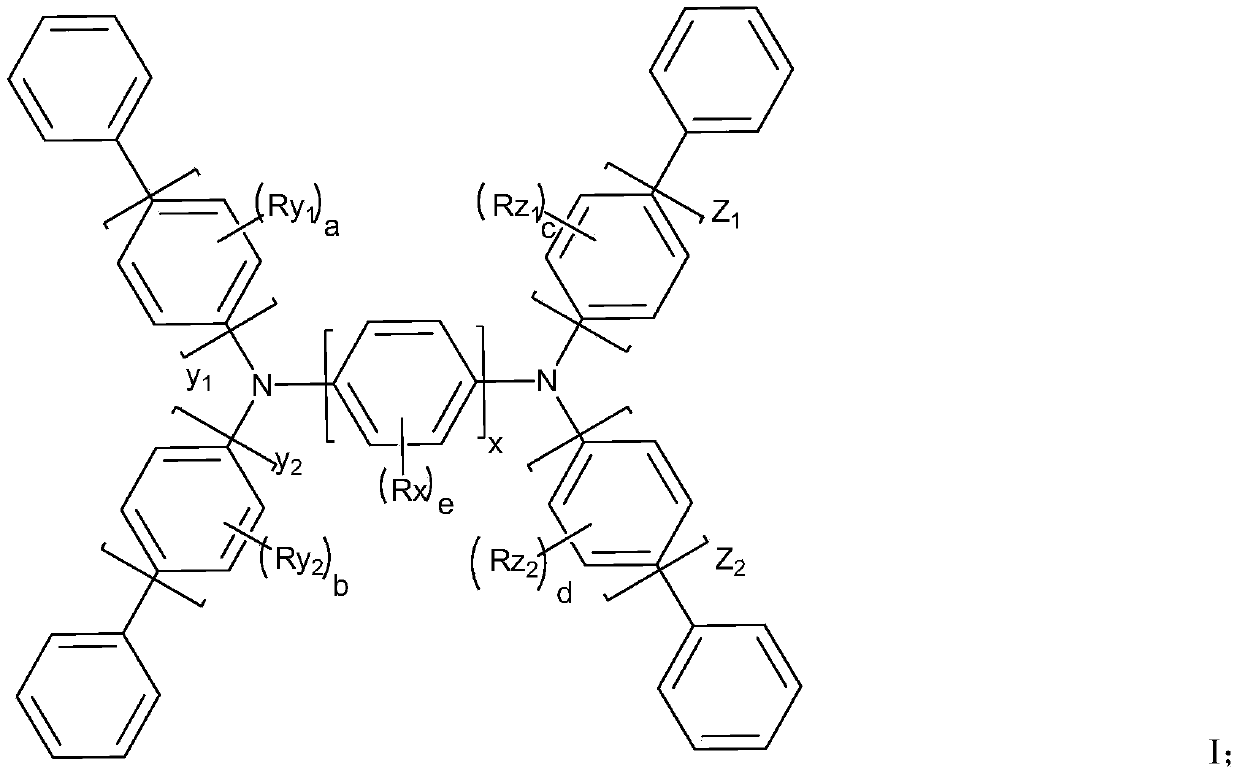
Aromatic amine derivative and organic electroluminescent device thereof
A luminescent and electromechanical technology, applied in the field of aromatic amine compounds and organic electroluminescent devices, can solve the problems of easy crystallization and high glass transition temperature of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0079] Intermediate Synthesis Example 2 (Synthesis of Intermediate M2)
[0080]
[0081] Under nitrogen protection, add 1mmol 2,2"-dimethyl-4,4"-dibromo-1,1':4',1"-terphenyl, 1mmol bis(p-terphenyl) to 50ml o-xylene solvent ) amine, 0.05% mmol of palladium acetate, 0.2 mmol tert-butylphosphine, 15 mmol sodium tert-butoxide, reacted at 100° C. for 4 hours, added 50 ml of water after cooling down to room temperature, extracted the organic layer, added methanol to the organic layer to precipitate Solid, recrystallized from toluene to give white powder, which is M2.
Embodiment 3
[0082] Intermediate synthesis embodiment 3 (synthesis of intermediate M3)
[0083]
[0084] Under nitrogen protection, add 1mmol 2,2"-difluoro-4,4"-dibromo-1,1':4',1"-terphenyl, 1mmol bis(p-terphenyl) to 50ml o-xylene solvent Amine, 0.05% mmol of palladium acetate, 0.2 mmol of tert-butylphosphine, 15 mmol of sodium tert-butoxide, react at 100°C for 4 hours, add 50 ml of water after cooling down to room temperature, extract the organic layer, add methanol to the organic layer to precipitate a solid , Recrystallized from toluene to give white powder, which is M3.
[0085] Intermediate synthesis embodiment 4 (synthesis of intermediate M4)
[0086]
[0087] Under nitrogen protection, add 1mmol 2,2'-difluoro-4,4"-dibromo-1,1':4',1"-terphenyl, 1mmol bis(p-terphenyl) to 50ml o-xylene solvent Amine, 0.05% mmol of palladium acetate, 0.2 mmol of tert-butylphosphine, 15 mmol of sodium tert-butoxide, react at 100°C for 4 hours, add 50 ml of water after cooling down to room tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com