Dabigatran etexilate mesylate content detection method
A technology of dabigatran etexilate mesylate and a detection method, applied in the field of medicine, can solve the problem of no dabigatran etexilate mesylate, etc., and achieve the effects of improving product quality and optimizing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Accurately weigh 10mg of dabigatran etexilate mesylate standard substance and dissolve it in a 10ml volumetric flask, dissolve it with methanol and dilute to the mark, shake well, and make a standard solution with a concentration of 1mg / ml.
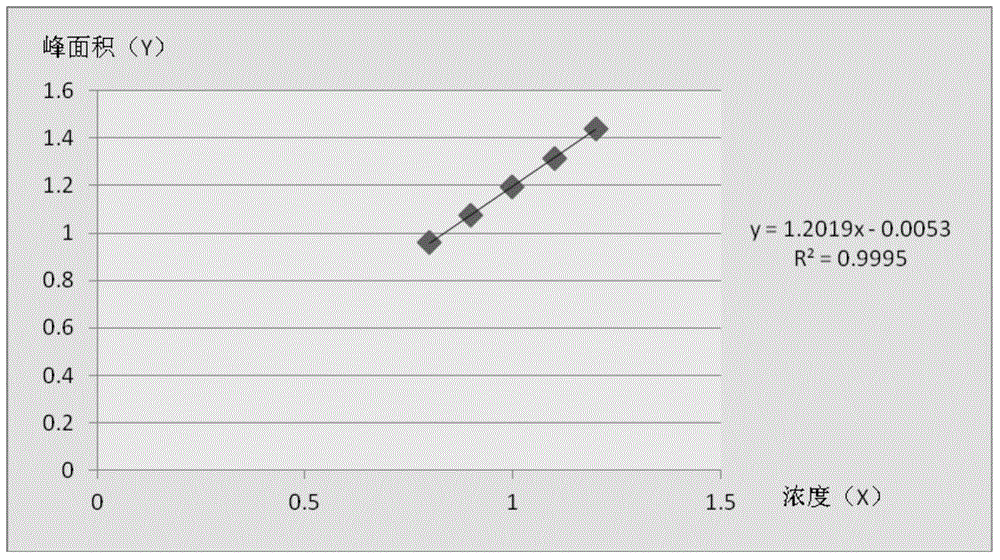
[0018] Accurately prepare yohimbine standard solution according to the above method, filter it through a 0.2 μm microporous filter head and inject it into a liquid chromatograph, according to the method determined in the present invention. The five yohimbine standard solutions with different concentrations are 0.8mg / ml, 0.9mg / ml, 1.1mg / ml and 1.2mg / ml respectively. Take the peak area (Y) as the ordinate, and the sample concentration (X) as the abscissa to carry out linear regression, get r=0.999, the linear relationship is very good, see image 3 .
[0019] Among them, chromatographic column: phenomenonexGeminiC18 (150×4.6mm, 5μm); mobile phase: A: 0.01mol / L ammonium acetate aqueous solution, acetic acid to adjust the pH to 6.5; B...
Embodiment 2
[0028] Reference substance solution: Accurately weigh an appropriate amount of dabigatran etexilate mesylate reference substance (purchased from sigma), dissolve it with methanol, and constant volume to make a solution containing 1 mg of dabigatran etexilate mesylate per 1 ml;
[0029] Need testing solution: Accurately take dabigatran etexilate mesylate test sample appropriate amount, dissolve with methanol, constant volume, make the solution that contains test sample 1mg in every 1ml approximately. (The test product is the product of the unit's synthesis room)
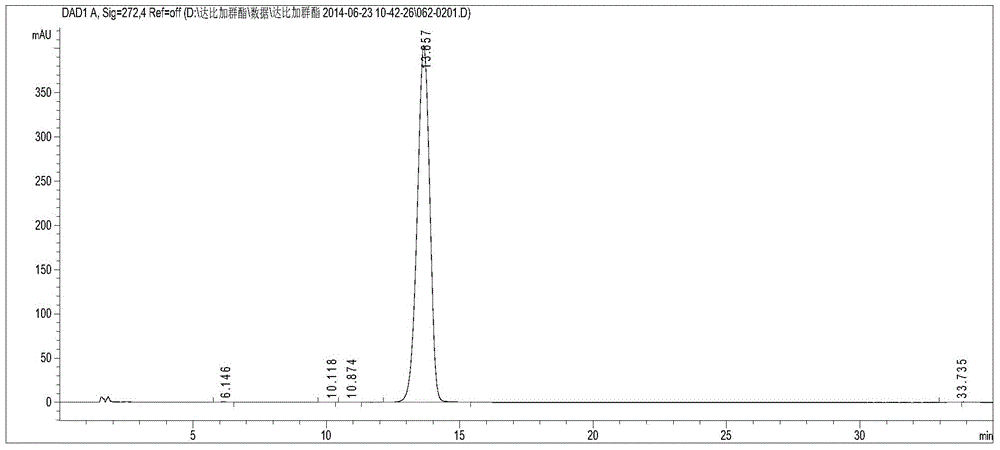
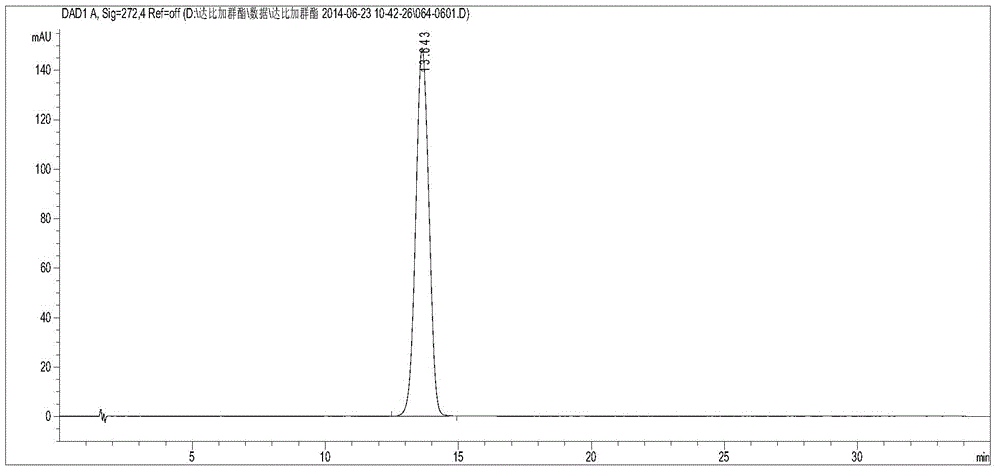
[0030] Take 10 μl each of the control solution and the test solution, inject them into the chromatograph, record the chromatogram, and calculate the peak area according to the external standard method to obtain the labeled amount of dabigatran etexilate mesylate.
[0031] Use the optimized chromatographic conditions in Example 1 to check, wherein, figure 1 Be the chromatogram of dabigatran etexilate mesylate test sam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com