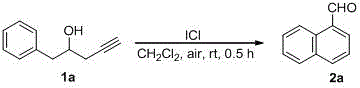Synthesizing method of fused-ring aryl substituted formaldehyde compound
A fused-ring aryl group and synthesis method technology, which is applied in the field of synthesis of aldehyde compounds, can solve the problems of difficult control of regioselectivity, difficulty in obtaining raw materials, and low practical value, and achieve a wide application range, simple operation, and mild conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015]
[0016] Add 1a (0.5mmol, 80mg) and dichloromethane (2mL) to a 10mL round bottom flask, then add ICl (1mmol, 1.0mL (1MinCH 2 Cl 2 )). After stirring the reaction at room temperature in an air atmosphere for 0.5 hours, the reaction was quenched by adding 2 mL of saturated sodium thiosulfate solution, extracted with dichloromethane (5 mL×3), and then the organic phase was washed with water and saturated brine in sequence, anhydrous sulfuric acid Sodium dry. Filtered, spin-dried, separated by silica gel column (petroleum ether / ethyl acetate=10 / 1) to obtain 1-naphthaldehyde 2a (50 mg, 64%) as light yellow liquid. The characterization data of this compound are as follows: 1 HNMR (400MHz, CDCl 3 )δ:7.58-7.64(m,2H),7.67-7.72(m,1H),7.92(d,J=7.6Hz,1H),7.98-7.99(m,1H),8.10(d,J=8.0Hz ,1H),9.26(d,J=8.0Hz,1H),10.40(s,1H); 13 CNMR (100MHz, CDCl 3 )δ: 124.9, 127.0, 128.5, 129.1, 130.6, 131.4, 133.7, 135.3, 136.7, 193.6; MS: m / z157 (MH) + .
Embodiment 2
[0018] According to the method described in Example 1, 1a (0.5mmol, 80mg) and acetonitrile (2mL) were added to a 10mL round bottom flask, and then ICl (1mmol, 1.0mL (1MinCH 2 Cl 2 )). After stirring the reaction at room temperature in an air atmosphere for 0.5 hours, the reaction was quenched by adding 2 mL of saturated sodium thiosulfate solution, extracted with dichloromethane (5 mL×3), and then the organic phase was washed with water and saturated brine in sequence, anhydrous sulfuric acid Sodium dry. Filtered, spin-dried, separated by silica gel column (petroleum ether / ethyl acetate=10 / 1) to obtain 1-naphthaldehyde 2a (6 mg, 8%) as light yellow liquid.
Embodiment 3
[0020] According to the method described in Example 1, 1a (0.5mmol, 80mg) and tetrahydrofuran (2mL) were added to a 10mL round bottom flask, and then ICl (1mmol, 1.0mL (1MinCH 2 Cl 2 )). After stirring the reaction at room temperature in an air atmosphere for 0.5 hours, the reaction was quenched by adding 2 mL of saturated sodium thiosulfate solution, extracted with dichloromethane (5 mL×3), and then the organic phase was washed with water and saturated brine in sequence, anhydrous sulfuric acid Sodium dry. Filtered, spin-dried, and separated on a silica gel column (petroleum ether / ethyl acetate=10 / 1) to obtain 1-naphthaldehyde 2a (5 mg, 7%) as a pale yellow liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com