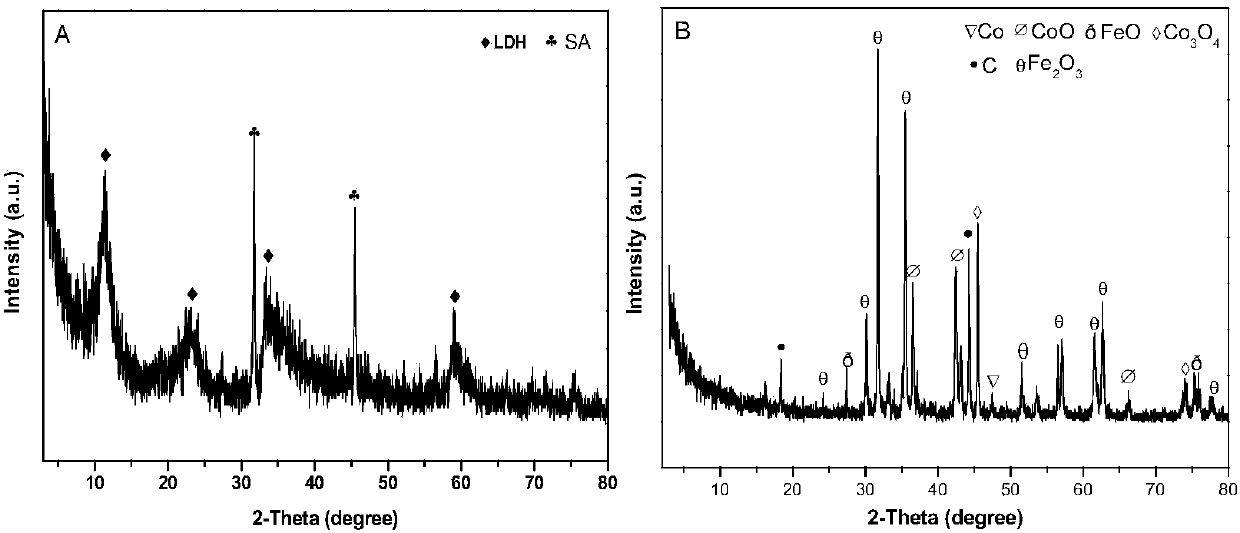
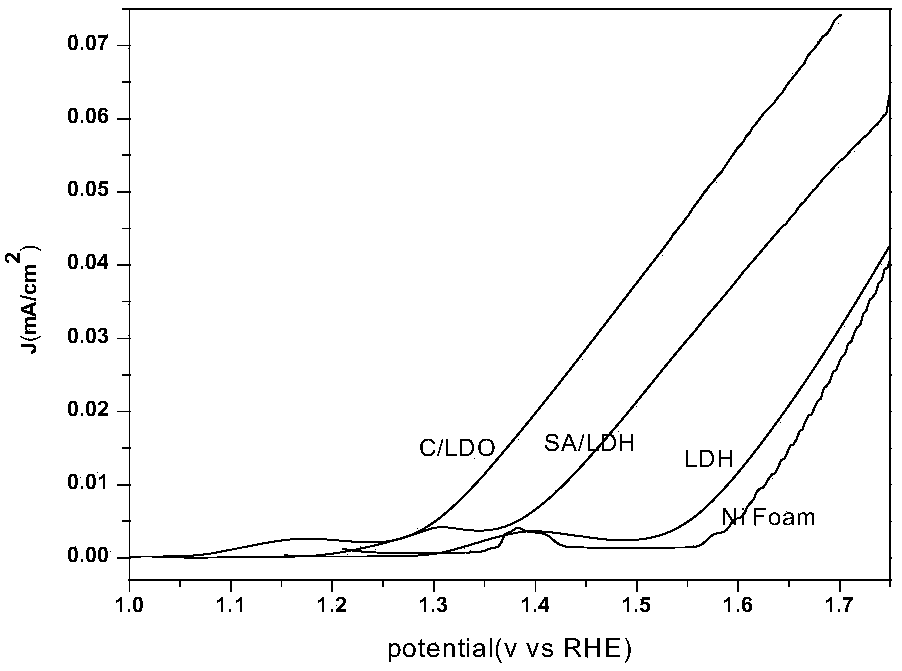
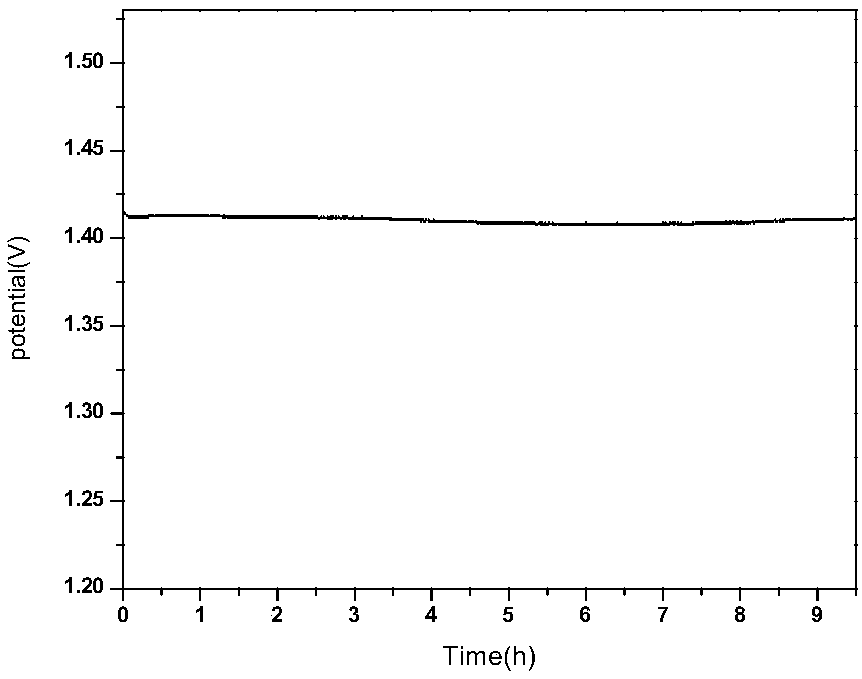
Biomass carbon/cobalt-iron double metal oxide bifunctional oxygen catalyst and its preparation method and application
A bimetallic oxide and biomass carbon technology, applied in structural parts, electrical components, battery electrodes, etc., to achieve the effects of low price, low preparation cost, and good methanol tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (a) Preparation of SA / LDH complex
[0032] Disperse a certain amount of SA in distilled water to make the concentration 2.5mg / ml, add cobalt chloride hexahydrate and ferric chloride hexahydrate into 150mL deionized water at a molar ratio of 3:1, so that the total concentration of metal ions is 0.016mol / L, stir and dissolve completely, then slowly add it dropwise into the SA solution, stir for 4h and then centrifuge at 3800rpm for 10min, collect the precipitate and redisperse it in 150mL deionized water, then add 1.5mol / L NaOH dropwise at a constant speed, adjust the pH of the reaction solution to 8. Transfer the above mixed solution into a reaction kettle, conduct a hydrothermal reaction at 130°C for 24 hours, wash the reaction solution three times with deionized water and ethanol in turn after centrifugation, and dry it to form the SA / LDH complex;
[0033] (b) Preparation of C / LDO bifunctional oxygen catalyst
[0034] Grind the SA / LDH composite obtained in step (a) i...
Embodiment 2
[0036] (a) Preparation of SA / LDH complexes
[0037] Disperse a certain amount of SA in distilled water to make its concentration 5mg / ml, and add cobalt chloride hexahydrate and ferric chloride hexahydrate into 150mL deionized water in a molar ratio of 3:1, so that the total concentration of metal ions is 0.016mol / L, slowly add dropwise to the SA solution after stirring to dissolve completely, stir for 4h, centrifuge at 3800rpm for 10min, collect the precipitate and re-disperse it in 150mL deionized water, then dropwise add 1.5mol / L NaOH at a uniform speed to adjust the pH of the reaction solution to 8, The above mixed solution was transferred to the reaction kettle, and hydrothermally reacted at 130°C for 24 hours. After centrifugation, the reaction solution was washed three times with deionized water and ethanol in turn, and the SA / LDH complex was obtained after drying;
[0038] (b) Preparation of C / LDO catalyst
[0039] Prepared according to the method and conditions of st...
Embodiment 3
[0042] (a) Preparation of SA / LDH complexes
[0043] Disperse a certain amount of SA in distilled water to make its concentration 10mg / ml, and add cobalt chloride hexahydrate and ferric chloride hexahydrate into 150mL deionized water at a molar ratio of 3:1, so that the total concentration of metal ions is 0.016mol / L, slowly add dropwise to SA solution after stirring to dissolve completely, centrifuge at 3800rpm for 10min after stirring for 4h, collect the precipitate and redisperse it in 150mL deionized water, and then dropwise add 1.5mol / L NaOH at a uniform speed to adjust the pH of the reaction solution to 8 , the above mixed solution was transferred into the reaction kettle, hydrothermally reacted at 130 ° C for 24 hours, the reaction solution was centrifuged and washed with deionized water and ethanol three times in turn, and it was the SA / LDH complex after drying;
[0044] (b) Preparation of C / LDO catalyst
[0045] Prepared according to the method and conditions of step...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com