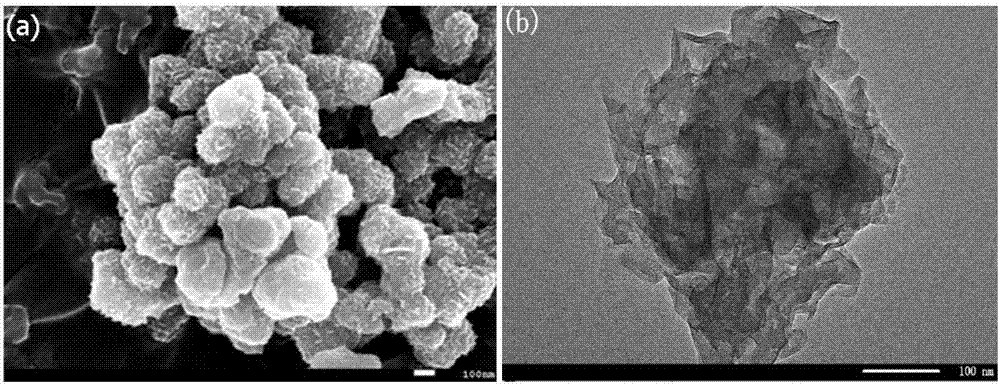
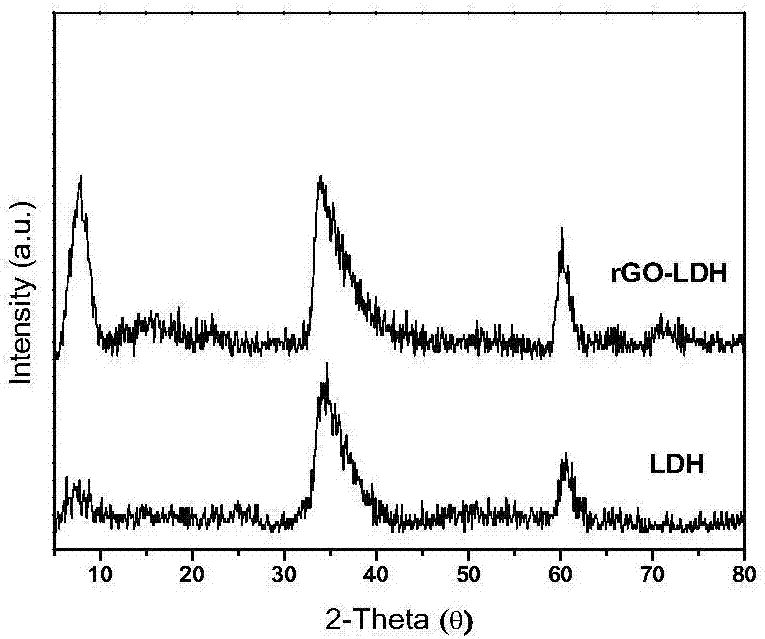
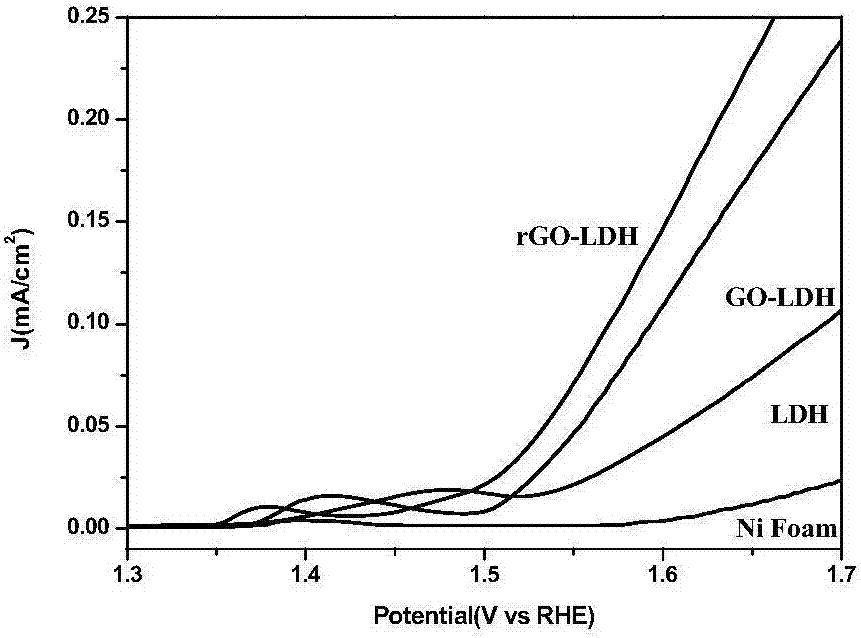
Graphene/nickel-iron hydrotalcite bifunctional oxygen catalyst and its preparation method and application
A technology of oxygen catalyst and hydrotalcite, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, chemical instrument and method, etc., to achieve low preparation cost, good OER and ORR activity, easy to purchase and the effect of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (a) Preparation of GO / LDH complex
[0033] Disperse a certain amount of GO in ethylene glycol to make the concentration 1.0mg / mL, ultrasonically disperse for 1 hour, and centrifuge at 3000rpm for 10min to remove the unstripped GO to obtain the stripped GO dispersion. Take 20mL of the dispersion and press 3: Add nickel chloride hexahydrate and ferric chloride hexahydrate to it at a molar ratio of 1, so that the total concentration of metal ions is 0.04mol / L, stir to make it dissolve completely, then slowly add 0.6g sodium dodecylsulfonate, stir Make it completely dissolved under certain conditions, then drop into 10mL ethylene glycol solution containing 0.16g NaOH at a constant speed, transfer the mixed solution into the reaction kettle, react at 180°C for 24h, centrifuge the reaction solution, and wash it with deionized water and ethanol respectively Three times, the GO / LDH complex is obtained after drying;
[0034] (b) Preparation of rGO / LDH oxygen catalyst
[0035] ...
Embodiment 2
[0037] (a) Preparation of GO / LDH complex
[0038] Disperse a certain amount of GO in ethylene glycol to make the concentration 0.5mg / mL, ultrasonically disperse for 1 hour, and centrifuge at 3000rpm for 10min to remove unstripped GO to obtain a stripped GO dispersion. Take 20mL of the dispersion and press 3: Add nickel chloride hexahydrate and ferric chloride hexahydrate to it at a molar ratio of 1, so that the total concentration of metal ions is 0.04mol / L, stir to make it dissolve completely, then slowly add 0.6g sodium dodecylsulfonate, stir Make it completely dissolved under certain conditions, then drop into 10mL ethylene glycol solution containing 0.16g NaOH at a constant speed, transfer the mixed solution into the reaction kettle, react at 180°C for 24h, centrifuge the reaction solution, and wash it with deionized water and ethanol respectively Three times, the GO / LDH complex is obtained after drying;
[0039] (b) Preparation of rGO / LDH oxygen catalyst
[0040] Prepar...
Embodiment 3
[0042] (a) Preparation of GO / LDH complex
[0043] Disperse a certain amount of GO in ethylene glycol to make the concentration 1.0mg / mL, ultrasonically disperse for 1 hour, and centrifuge at 3000rpm for 10min to remove the unstripped GO to obtain the stripped GO dispersion. Take 20mL of the dispersion and press 3: Add nickel chloride hexahydrate and ferric chloride hexahydrate to it at a molar ratio of 1, so that the total concentration of metal ions is 0.04mol / L, stir to dissolve it completely, then slowly add 0.2g sodium dodecylsulfonate, stir Make it completely dissolved under certain conditions, then drop into 10mL ethylene glycol solution containing 0.16g NaOH at a constant speed, transfer the mixed solution into the reaction kettle, react at 180°C for 24h, centrifuge the reaction solution, and wash it with deionized water and ethanol respectively Three times, the GO / LDH complex is obtained after drying;
[0044] (b) Preparation of rGO / LDH oxygen catalyst
[0045] Prepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com