A kit for genetic detection of Middle East Respiratory Syndrome Coronavirus
A technology for respiratory syndrome and coronavirus, applied in the detection/testing of microorganisms, resistance to vector-borne diseases, biochemical equipment and methods, etc., can solve the problem of no cross-reactivity of respiratory viruses, and achieve the time required for detection Effect of shortening, ensuring specificity and accuracy, and reducing workload
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
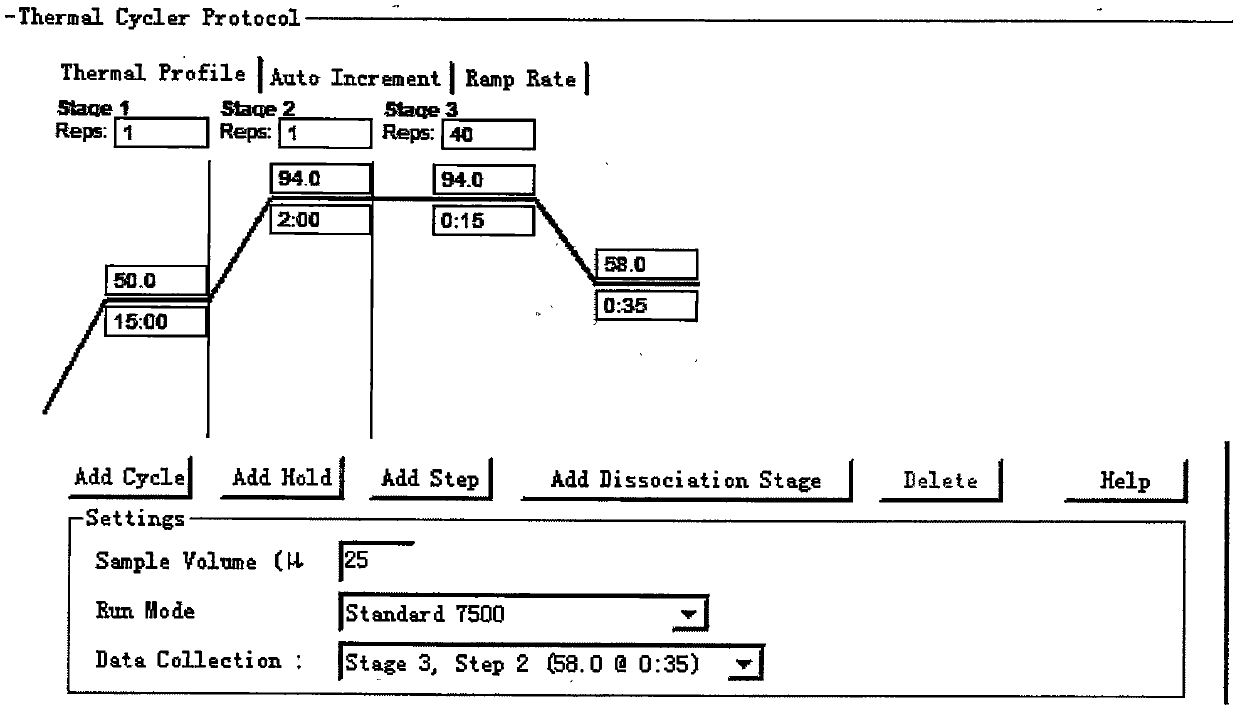
[0023] The preparation and use of embodiment 1 Middle East respiratory syndrome coronavirus gene detection kit
[0024] 1. Prepare a kit including the following components
[0025] 1 tube of primer-probe mixture (25 μl / tube), 1 tube of RT-PCR reaction solution (125 μl / tube), 1 tube of RT-PCR enzyme system (75 μl / tube), 1 tube of positive quality control (200 μl / tube), Negative quality control (200μ / l tube) 1 tube, DEPC H 2 O (2000μl / tube) 1 tube.
[0026] 2. Preparation of target gene recombinant plasmid
[0027] The entrusted company synthesized and purified the in vitro transcribed RNA of UPE and N genes of Middle East respiratory syndrome coronavirus, measured the concentration by ultraviolet spectrophotometer, and then calculated the RNA copy number, and used DEPC H for the two kinds of RNA 2 O were diluted to 1.0 × 10 5 copies / mL~1.0×10 2 copies / mL of the standard.
[0028] 3. Real-time fluorescent quantitative PCR amplification and detection
[0029] 3.1 Reagent p...
Embodiment 2
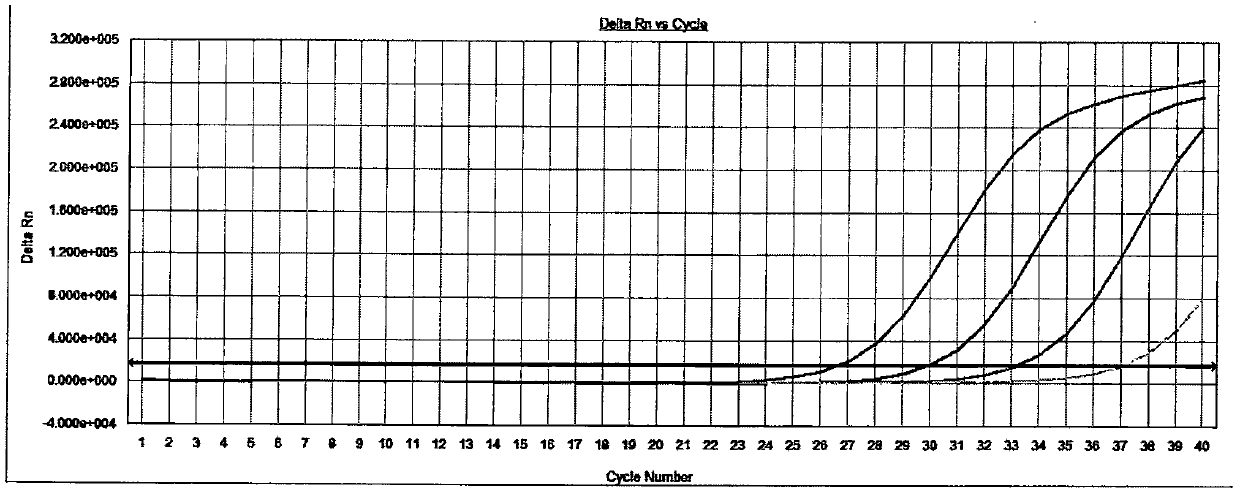
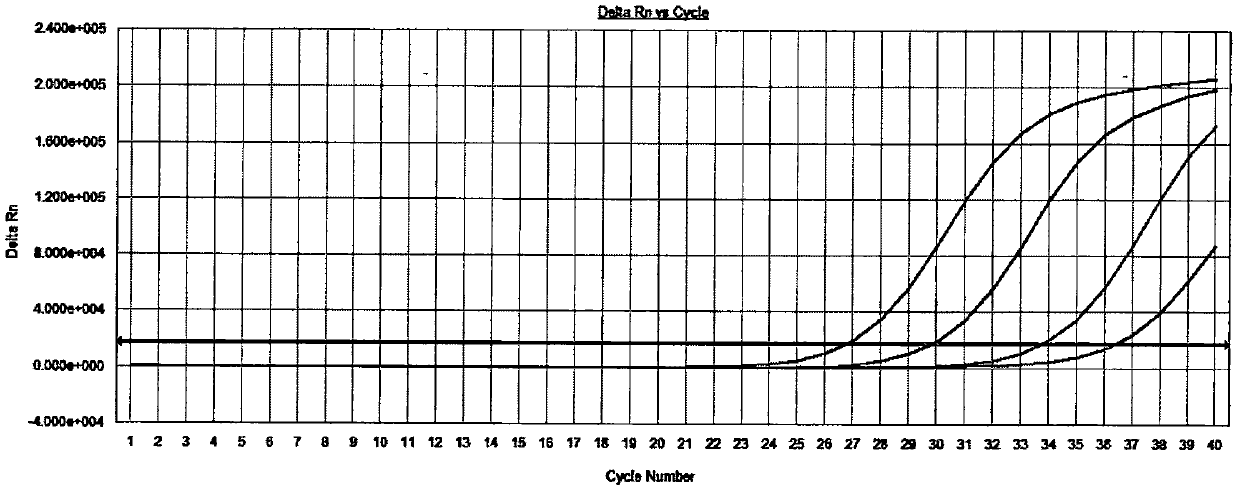
[0039] The detection of embodiment 2 specific sample
[0040] Select HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU13, H3N2 (influenza virus), H1N1 (influenza virus), Adenovirus (adenovirus) type 5, RSV (respiratory syncytial virus), EV71 (enterovirus ), PIV-1 (parainfluenza virus-1 type), PIV-2 (parainfluenza virus-2 type), PIV-3 (parainfluenza virus-3 type), PIV-4 (parainfluenza virus-4 type), Rhinovirus, HSV-1 (herpes simplex virus), Epstein-Barr virus, human cytomegalovirus, measles virus each positive sample 1 case is used as specific sample, carries out nucleic acid extraction to all samples, PCR amplification and result analysis steps refer to embodiment 1, and carry out the detection of negative and positive quality control products at the same time.
[0041] Test result: the amplification curve of the negative quality control product is not S-shaped (see attached Figure 4 ), the amplification curve of the positive quality control product is an obvious S-shaped curve (se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com