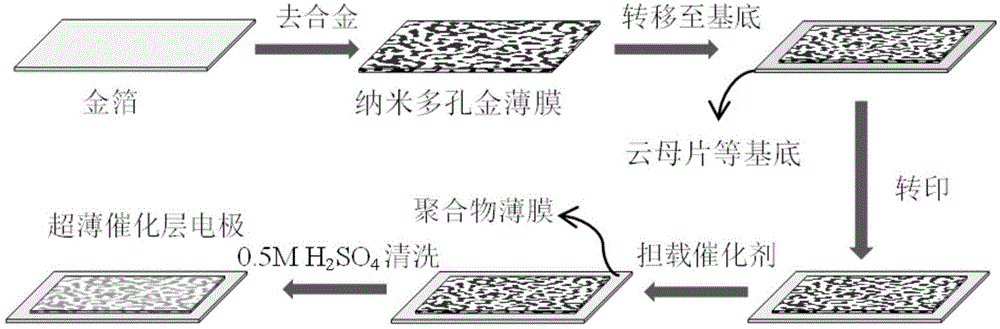
Method for preparing fuel cell thin film electrode by using nanoporous gold
A nanoporous gold and fuel cell technology, applied in battery electrodes, circuits, electrical components, etc., can solve the difficulty of increasing the surface modification of nanoporous gold, the limitation of nanoporous gold films, and the fragility of nanoporous gold films. Improved loading efficiency, high catalyst dispersion, and less cracking effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
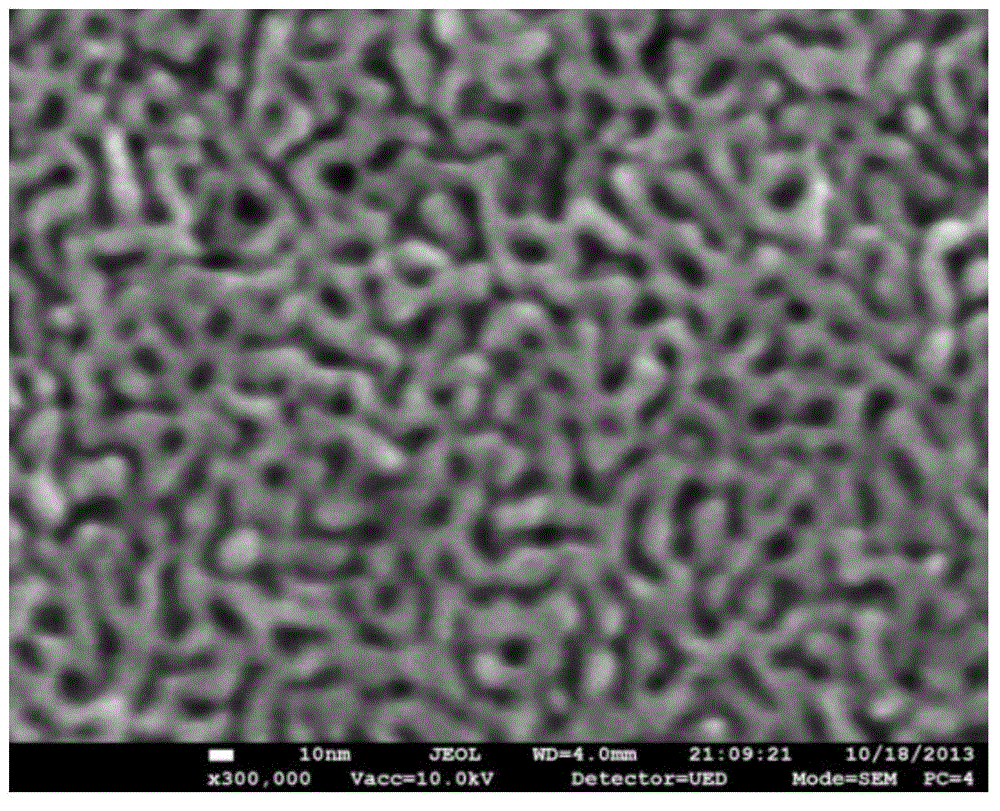
[0033] Using 12K gold foil as raw material, cut the gold foil into a suitable size, spread the gold foil on the surface of deionized water, and transfer it to diluted nitric acid (the volume ratio of nitric acid and deionized water is 2:1), and control the reaction time The as-prepared nanoporous gold was labeled as NPG(30). The nanoporous gold film (NPG, nanoporous goldfilm) obtained after dealloying is transferred to deionized water, and the nanoporous gold film is spread on the surface of deionized water at this time; replace deionization 3-4 times, remove the nanoporous gold film. nitric acid; the nanoporous gold film is transferred to a mica sheet (size 2.5cm × 4.0cm), and dried at room temperature; the Nafion211 film is covered on the side with the nanoporous gold film, and hot-pressed at 150°C and 5MPa 3min; carefully remove the mica sheet, and wash the obtained Nafion membrane with deionized water. The pore size test shows that the porosity of the prepared nanoporous ...
Embodiment 2
[0037] Using 12K gold foil as raw material, cut the gold foil into a suitable size, spread the gold foil on the surface of deionized water, and transfer it to diluted nitric acid (the volume ratio of nitric acid and deionized water is 1:1), and control the reaction time The as-prepared nanoporous gold was labeled as NPG(30). Transfer the nanoporous gold film obtained after dealloying to deionized water, and at this time, the nanoporous gold film spreads on the surface of the deionized water; replace the deionized 3-4 times to remove the nitric acid on the nanoporous gold film; The porous gold film was transferred to a mica sheet (size 2.5cm*4.0cm), and dried at room temperature; the Nafion211 film was covered on the side with the nanoporous gold film, and hot-pressed at 150°C and 6MPa for 1.5min; carefully Remove the mica sheet and wash the Nafion membrane at this point with deionized water.
[0038] Pt was deposited on nanoporous gold films by CV electrodeposition. The spec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com