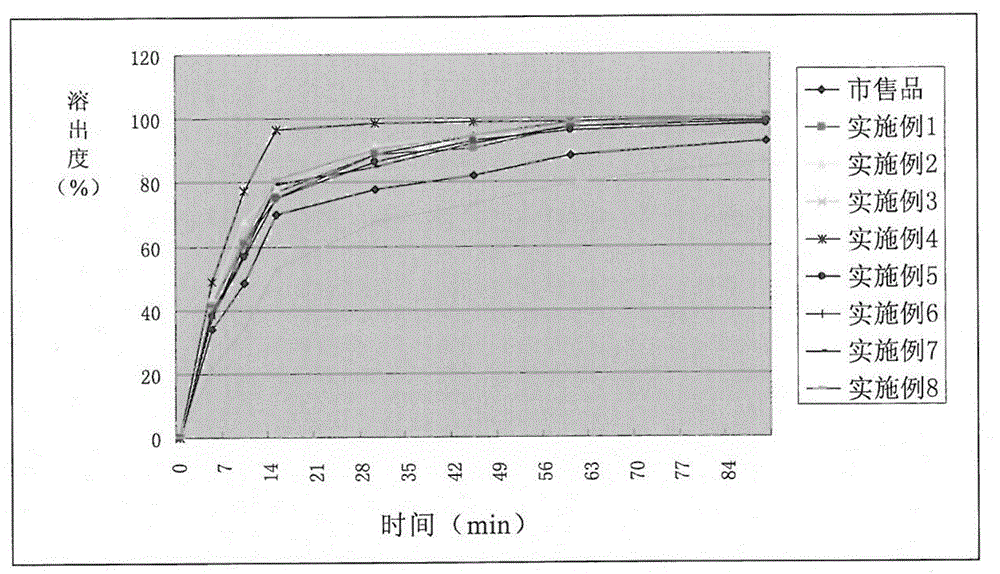
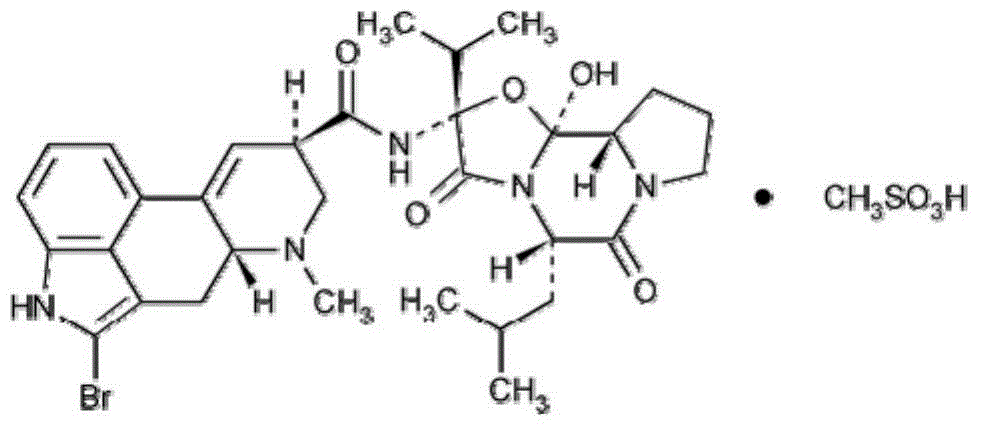
Method for preparing bromocriptine mesylate tablets by virtue of novel production process and application of method
A technology of bromocriptine mesylate tablets and bromocriptine mesylate, which can be used in metabolic diseases, antineoplastic drugs, endocrine system diseases, etc., can solve the problems of degradation and increase in impurities, high production costs, and relatively different tablet contents. Large and other problems, to achieve the effect of small content difference, improved stability, and reduced impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] prescription:
[0050]
[0051] Preparation method: Dissolve bromocriptine mesylate in 96% ethanol at room temperature to make a solution. The active ingredient solution is sprayed onto the homogenized and preheated powder mixture (mixture made by mixing lactose, corn starch and croscarmellose sodium), and the organic solvent ethanol is removed by drying. The powder mixture containing the active ingredient bromocriptine mesylate is granulated using a binder solution (made with cornstarch and purified water). Add glidants colloidal silicon dioxide and talc to the granules, then sieve the mixed granules, add magnesium stearate, lubricate the sieved granules, and compress into tablets. A tablet with a product specification of 2.5 mg was obtained.
Embodiment 2
[0053] prescription:
[0054]
[0055] Preparation method: Dissolve bromocriptine mesylate in 96% ethanol at room temperature to make a solution. The active ingredient solution is sprayed onto the homogenized and preheated powder mixture (mixture made by mixing lactose, corn starch and croscarmellose sodium), and the organic solvent ethanol is removed by drying. The powder mixture containing the active ingredient bromocriptine mesylate is granulated using a binder solution (made with cornstarch and purified water). Add glidants colloidal silicon dioxide and talc to the granules, then sieve the mixed granules, add magnesium stearate, lubricate the sieved granules, and compress into tablets. A tablet with a product specification of 0.8 mg was obtained.
Embodiment 3
[0057] prescription:
[0058]
[0059] Preparation method: Dissolve bromocriptine mesylate in 96% ethanol at room temperature to make a solution. The active ingredient solution is sprayed onto the homogenized and preheated powder mixture (mixture made by mixing lactose, corn starch and croscarmellose sodium), and the organic solvent ethanol is removed by drying. The powder mixture containing the active ingredient bromocriptine mesylate is granulated using a binder solution (made with cornstarch and purified water). Add glidants colloidal silicon dioxide and talc to the granules, then sieve the mixed granules, add magnesium stearate, lubricate the sieved granules, and compress into tablets. A tablet with a product specification of 2.5 mg was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com