Preparation method and kit of bispecific chimeric antigen receptor gene modified natural killer cells
A technology of chimeric antigen receptors and natural killer cells, applied to cells modified by introducing foreign genetic material, receptors/cell surface antigens/cell surface determinants, chemical instruments and methods, etc. Poor antitumor activity, low antitumor activity, insufficient immune function against tumors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
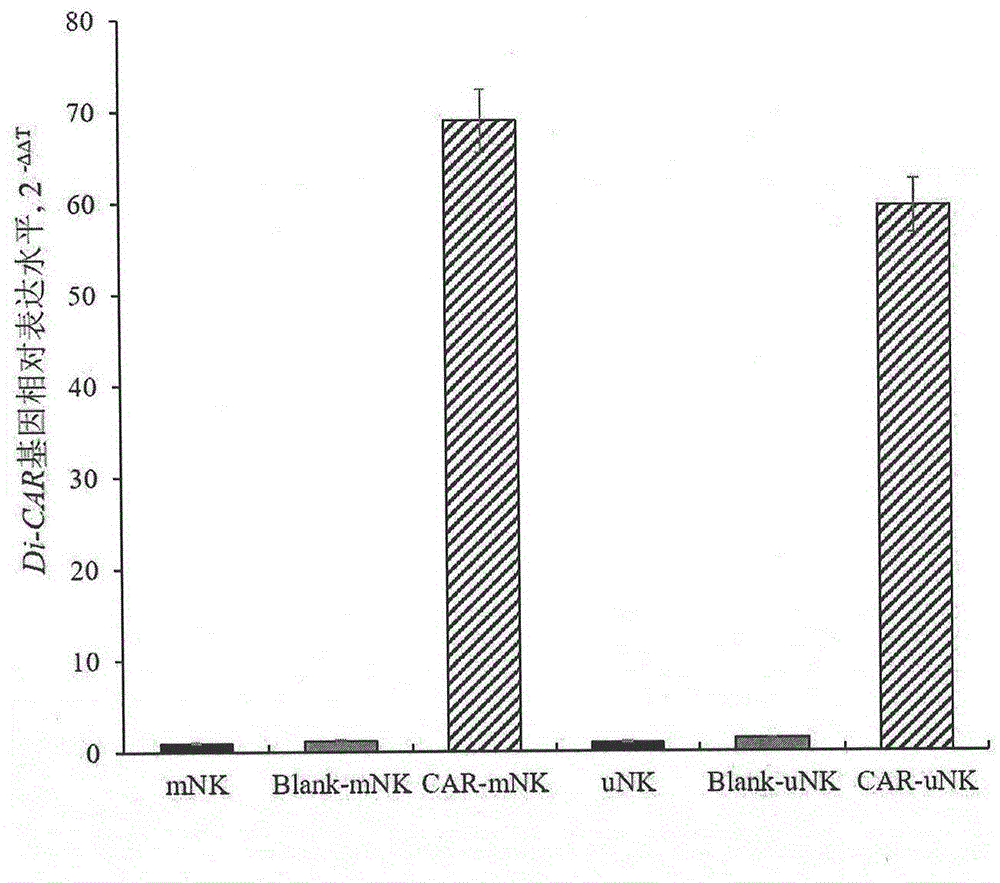
[0040] The invention relates to a method for preparing natural killer cells genetically modified with bispecific chimeric antigen receptors. Human natural killer cells are transfected, and the bispecific chimeric antigen receptor gene is highly expressed, which can specifically bind to tumor cells expressing signaling lymphocyte activation molecule family member 7 and fibronectin variants, and inhibit the inhibitory effect of natural killer cells. The body expression avoids the immune evasion of tumor cells; simultaneously activates the first signal and co-stimulatory signal to trigger anti-tumor cytotoxic activity, and activates a strong anti-tumor response in vivo and in vitro.
[0041] The SLAMF7 and FNv single-chain antibodies in the bispecific chimeric antigen receptor gene of the present invention specifically bind to the SLAMF7 and FNv antigens expressed by malignant tumor cells, so that NK cells can produce anti-tumor targeting specificity, preferably targeting melanin ...
Embodiment 1
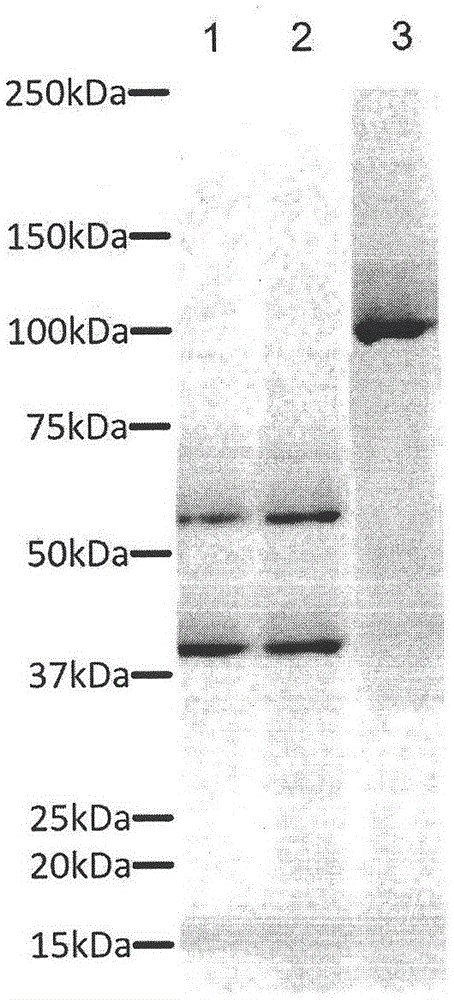
[0075] Construction of recombinant Di-CAR gene and preparation of lentivirus
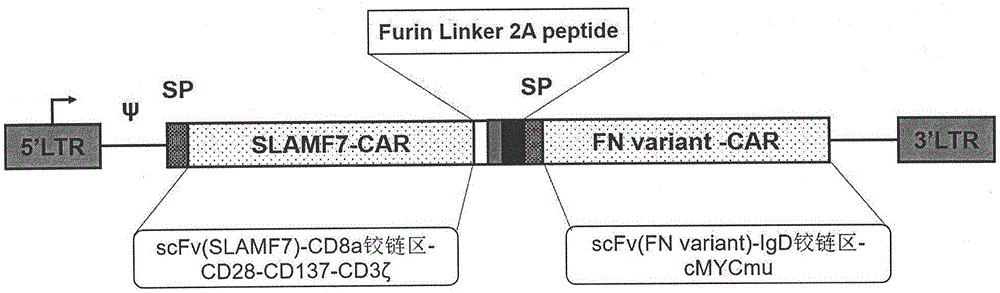
[0076] Human IFNγ-signal peptide-scFv(SLAMF7)-CD8a hinge region-CD28-CD137-CD3ζ intracellular region, and scFv(FNv)-IgD hinge region-c-Myc mutant gene through Furin cleavage site and linker sequence Fragment composition, the 362nd leucine of the c-Myc mutant is mutated to valine, namely c-Myc-L / V, see figure 1 , which is the schematic diagram of Di-CAR fusion protein.
[0077] The human IFNγ-signal peptide-scFv (SLAMF7)-CD8a hinge region-CD28-CD137-CD3ζ intracellular region, and the scFv (FNv)-IgD hinge region-c-Myc mutant were passed through the Furin cleavage site and linking sequence (Linker ) are linked together to form a recombinant gene sequence, which is obtained by chemical synthesis to form a complete cDNA of the bispecific chimeric antigen receptor gene, that is, Di-CAR;
[0078] After the target DNA fragment of Di-CAR and the pUC229 vector HindIII / BamHI were double-digested, under the a...
Embodiment 2
[0083] Preparation of Natural Killer Cells Genetically Modified with Bispecific Chimeric Antigen Receptor
[0084] Collect 30ml of patients with advanced melanoma and uveal melanoma (with informed consent), add an equal amount of 1×PBS to dilute, gently superimpose on the lymphocyte separation medium along the wall of the centrifuge tube to form a clear interface, and centrifuge at 2000rpm at room temperature After 20 minutes, the PBMCs in the interface layer were sucked and washed twice with 1×PBS (pH 7.4). After counting orchids, resuspend with RPMI1640 medium and dilute to 3×10 6 Cells / ml, resuspended with RPMI1640 medium and diluted to 3×10 6 Cells / ml, supplemented with 50ng / mlIL-15, 20ng / mlIL-12, 20ng / mlIL-18, 100IU / mlIL-2 and 10% autologous serum, 1.2ml per well was added to mouse anti-human CD16-coated 24 In an orifice plate, at 37°C, containing 5% CO 2 Continue culturing in the incubator until day 5. NK cells were harvested by centrifugation on day 5 and counted by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com