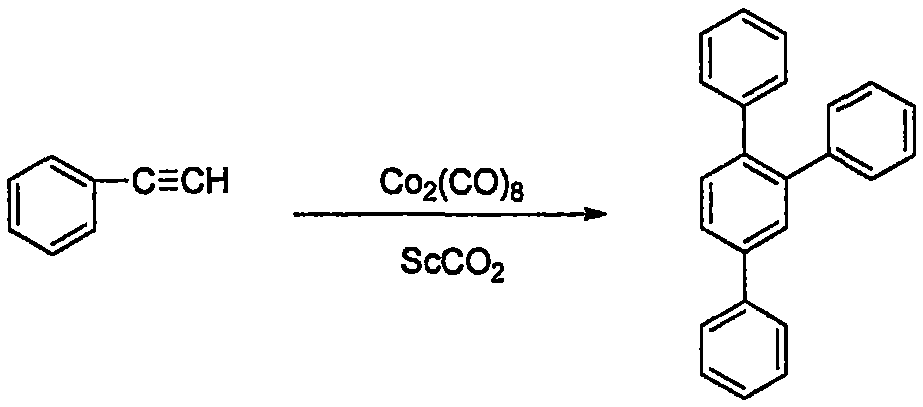Method of preparing 1,2,4-triphenylbenzene in supercritical CO2
A carbon dioxide, triphenylbenzene technology, applied in chemical instruments and methods, organic chemistry, hydrocarbons, etc., can solve the problems of few reports on alkyne cycloaddition, high reaction temperature, high pressure, long reaction time, etc. , to achieve the effect of high yield, short reaction time and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 0.2ml, 1.82mmol of phenylacetylene and 47mg, 0.14mmol of dicobalt octacarbonyl into a 50ml reaction vessel lined with polytetrafluoroethylene, and put in a suitable magnet. Use CO 2 The air was replaced twice, the reactor was preheated in an oil bath at 50°C for 20 minutes, and carbon dioxide was pumped in to 8MPa. Turn on the stirring, raise the temperature of the reactor to 80°C, and react for 8 hours.
[0029] After the reaction, the reactor was cooled to room temperature. Slowly vent the carbon dioxide to atmospheric pressure. The autoclave was washed with dichloromethane, and the entire reaction mixture was collected into a 100 ml round bottom flask. The excess solvent dichloromethane was distilled off under reduced pressure to obtain a crude product. The crude product was separated by column chromatography (200-300 mesh silica gel) using petroleum ether and dichloromethane (V / V=2:1) as the eluent to obtain white solids 1, 2, 4 with a purity of more than ...
Embodiment 2
[0031] Add 0.2ml, 1.82mmol of phenylacetylene and 47mg, 0.14mmol of dicobalt octacarbonyl into a 50ml reaction vessel lined with polytetrafluoroethylene, and put in a suitable magnet. Use CO 2 The air was replaced twice, the reactor was placed in an oil bath at 50°C to preheat for 20 minutes, and carbon dioxide was pumped in to 10MPa. Turn on the stirring, raise the temperature of the reactor to 100°C, and react for 10 hours.
[0032] The post-treatment process was the same as in Example 1 to obtain white solid 1,2,4-triphenylbenzene with a purity greater than 99%, and the isolated yield was 70%.
Embodiment 3
[0034] Add 0.2ml, 1.82mmol of phenylacetylene and 47mg, 0.14mmol of dicobalt octacarbonyl into a 50ml reaction vessel lined with polytetrafluoroethylene, and put in a suitable magnet. Use CO 2 Replace the air twice, preheat the reactor, and pump carbon dioxide to 12MPa. Turn on the stirring, raise the temperature of the reactor to 120°C, and react for 12 hours.
[0035] The post-treatment process was the same as in Example 1, and a white solid 1,2,4-triphenylbenzene with a purity greater than 99% was obtained with an isolated yield of 60%.
[0036] Identification of 1,2,4-triphenylbenzene:
[0037] Infrared spectrum data IR(KBr)v / cm -1 : 3080, 3054, 3025 (v CH , Ph), 1599, 1472, 1450 (v C=C , Ph), 759, 697 (γ CH , Ph).
[0038] NMR data: 1 HNMR (500MHz, CDCl 3 , TMS): δ7.66-7.16 (m, 18H, Ph); 13 CNMR (125MHz, CDCl 3 , TMS): δ140.44, 140.06, 139.94, 139.54, 139.32, 138.50 (centeralPh), 130.07, 128.86, 128.83, 128.38, 127.79, 126.89, 126.86, 126.39, 126.10, 125.48, 12...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com