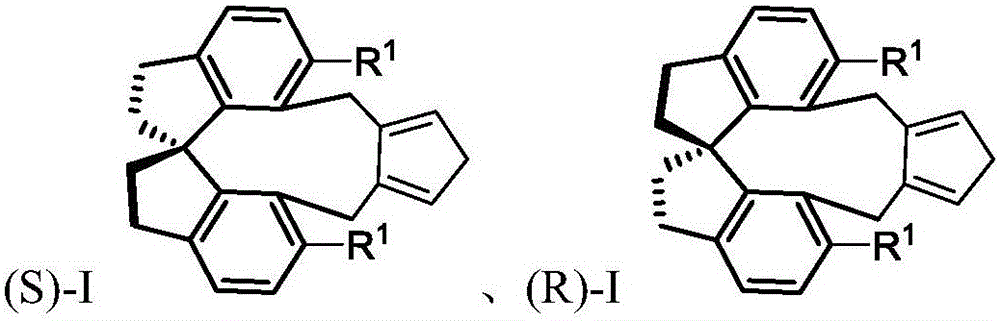
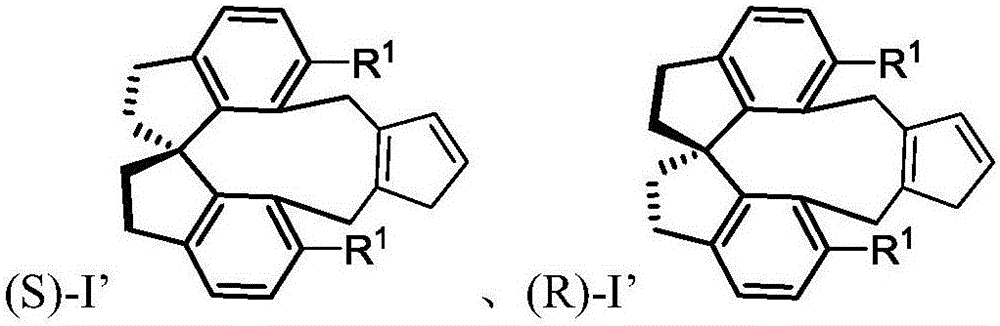
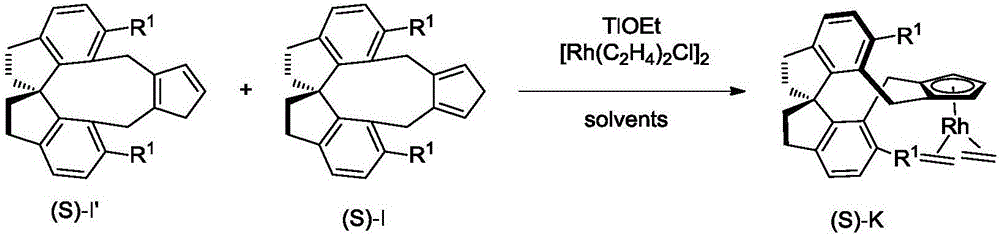
Spiro-framework-based cyclopentadiene compounds, rhodium complexes, and synthesis method and application thereof
A technology of cyclopentadiene and rhodium complexes, applied in the direction of organic compound/hydride/coordination complex catalysts, rhodium organic compounds, platinum group organic compounds, etc., can solve the problem of poor enantioselectivity control and skeleton Scarcity and other issues, to achieve high enantioselectivity and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Embodiment 1: Synthesis based on helical chiral cyclopentadiene compound (S)-K1:
[0100]
[0101] Synthesis of (S)-VI-1: Under argon protection, in a 100mL sealed tube, add (S)-VII (0.92g, 3mmol), Pd(OAc) 2 (134.7mg, 0.6mmol), iodobenzene acetate (2.42g, 7.5mmol), iodine (1.9g, 7.5mmol) and DMF (30mL), stirred and dissolved, heated to 100°C, and stirred for 36 hours. The reaction was cooled to room temperature, the solvent was removed under reduced pressure, and saturated Na 2 SO 3 (30 mL), then acidified to acidic with 1N HCl (30 mL), extracted anhydrous Na with DCM 2 SO 4 dry. After filtration, the solvent was distilled off under reduced pressure, and the crude product was separated by column chromatography (petroleum ether / ethyl acetate / acetic acid: 4 / 1 / 0.01).
[0102]
[0103] White solid, 70% yield, m.p.>300℃. 1 HNMR (400MHz, DMSO-d 6 )δ8.19(d, J=8.0Hz, 2H), 7.56(d, J=8.0Hz, 2H), 3.48-3.35(m, 4H), 2.95-2.87(m, 2H), 2.80-2.69(m ,2H); 13 CNMR (100MHz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com