Medicine composition for treating bone marrow hyperplasia and osteocarcinoma and application of medicine composition
A bone marrow proliferation and composition technology, which is applied in the direction of drug combination, boron compound active ingredients, bone diseases, etc., can solve the problems of unsatisfactory treatment effect and human side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The acquisition of embodiment 1 polypeptide
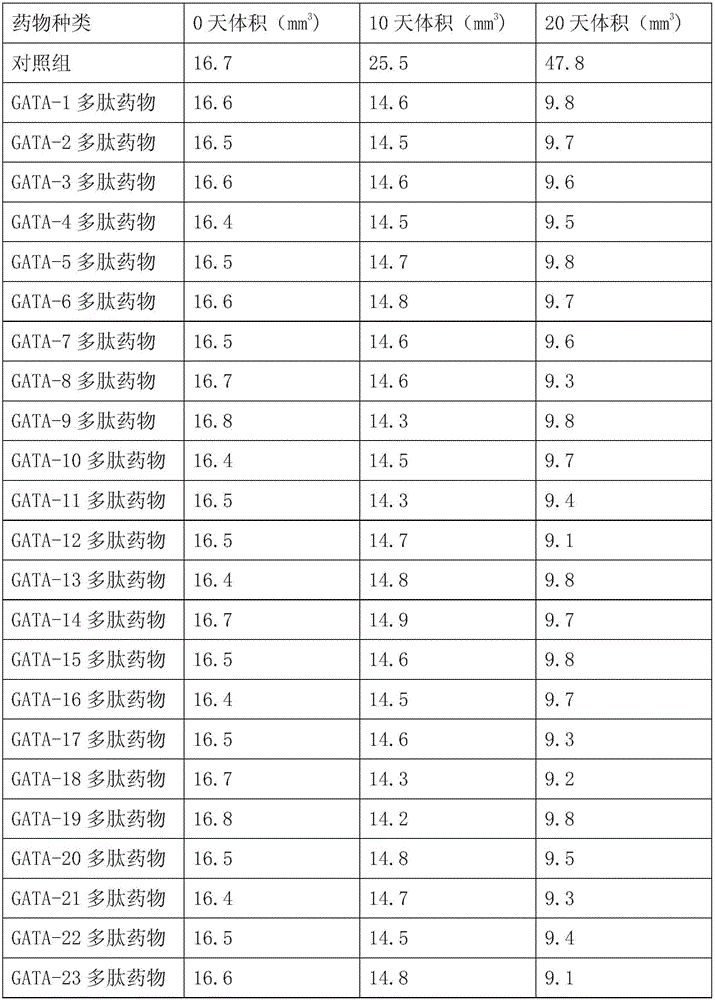
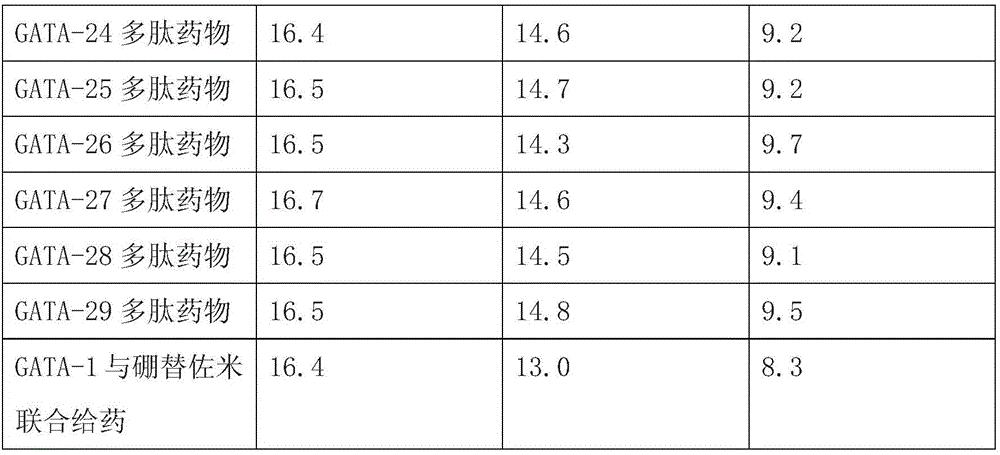
[0015] Weigh 2g of the leaves of Echinacea dwarf, mash and prepare PBS aqueous solution with a substrate concentration of 5%, adjust the pH to 8.0, add trypsin 4000U / g leaves, enzymolysis temperature is 50°C, and after 90min of enzymolysis, the enzyme The solution was rapidly heated to 90°C and kept for 20 minutes for enzyme inactivation treatment. Then quickly cooled to room temperature, centrifuged at 3000r / min for 15min, and took the supernatant. According to the ratio of 100 grams: 100ml, trypsin hydrolyzate was added to the wet macroporous adsorption resin DA201-C, kept on a constant temperature shaker at 25°C for 180min, the rotating speed was 150r / min, eluted with 75% ethanol solution for 120min, and collected different eluent for the time period. The small molecular weight bands were recovered, and a total of 78 small peptide sequences were obtained. It has been verified that 29 small peptides have the function of...
Embodiment 2
[0016] The preparation of embodiment 2 pharmaceutical compositions
[0017] Take 0.01 part of GATA-1 polypeptide, 1 part of starch, an appropriate amount of cyclamate or / and sucrose as sweetener, and 0.1 part of benzoic acid, mix well, pass through a 50-mesh sieve, sterilize, and pack.
[0018] GATA-2-29 polypeptides are also prepared into corresponding compositions according to the preparation method of GATA-1 polypeptide.
Embodiment 3
[0019] The mixing of embodiment 3 pharmaceutical composition and other preparations
[0020] Mix the GATA-1 polypeptide and bortezomib according to the ratio of 1:0.75, and then take the weight of the mixed drug as the basic number, then add 1 part of starch, appropriate amount of cyclamate or / and sucrose as sweetener, benzoic acid 0.1 portion, mixed evenly, passed through a 50-mesh sieve, sterilized, and packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com