Tripolymer TRAIL protein and application thereof
A trimer and protein technology, applied in the direction of peptide/protein components, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as difficult formation of trimer structures, and reduce immunogens Sex, increasing solubility and stability, improving the effect of enzymatic hydrolysis resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
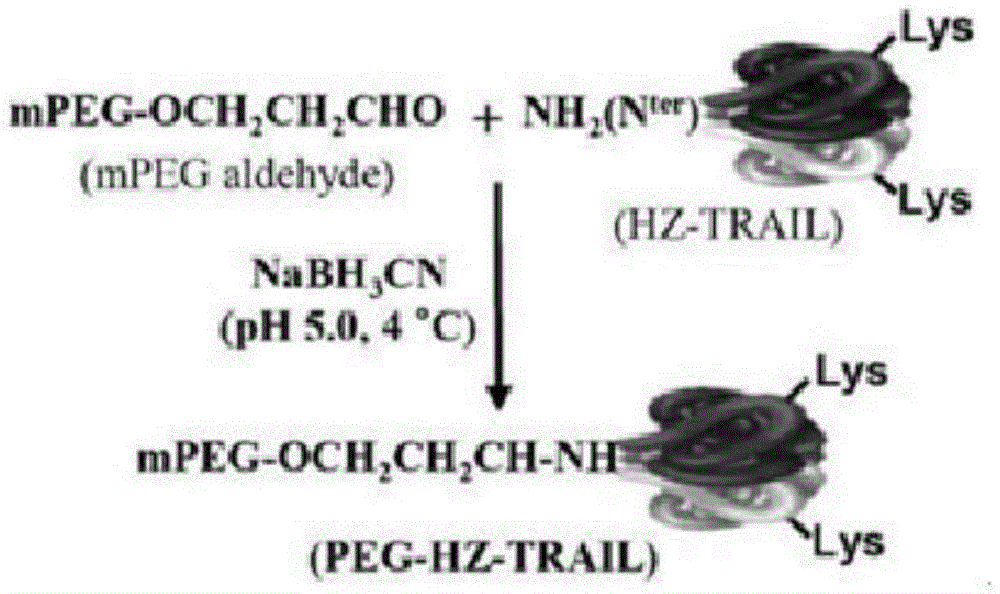
[0047] A trimeric TRAIL recombinant protein, such as figure 1 The schematic diagram of the preparation of PEG-HZ-TRAIL is shown, the inducing ligand is tumor necrosis factor-related apoptosis-inducing ligand TRAIL, and the preparation method is a chemical modification method, including the following steps:
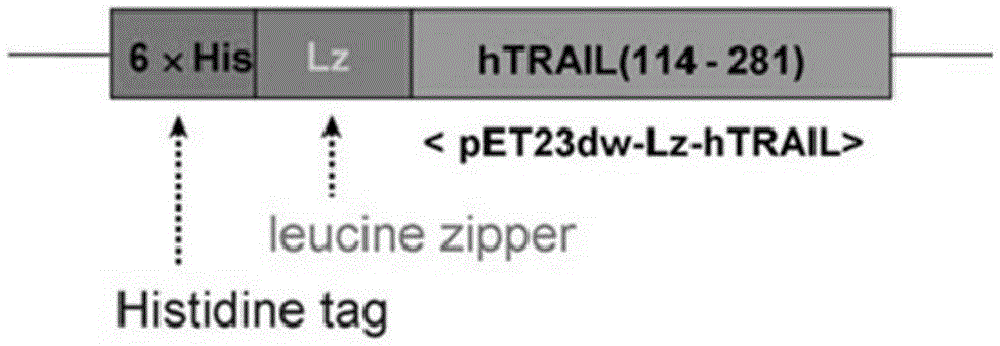
[0048] Step 1: Preparation of HZ-TRAIL: Gene sequence 114-281 in human TRAIL protein was cloned using pET expression vector to prepare recombinant gene with N-terminal histidine tag and isoleucine zipper motif. TRAIL recombinant protein of pET23dwpET23dw-His-ILZ-hTRAIL114-281, referred to as HZ-TRAIL, such as figure 2 The schematic diagram showing the preparation of HZ-TRAIL;
[0049] Step 2: Extraction and purification of HZ-TRAIL: Transform the HZ-TRAIL prepared in Step 1 into Escherichia coli, and in LB culture medium containing 1mmol / L isopropylthiogalactopyranoside (IPTG) at 25°C , amplified for 9 hours, and then recombinantly expressed HZ-TRAIL in Escherichia coli...
Embodiment 2
[0053] A trimeric TRAIL recombinant protein, such as figure 1 The schematic diagram of the preparation of PEG-HZ-TRAIL is shown, the inducing ligand is tumor necrosis factor-related apoptosis-inducing ligand TRAIL, and the preparation method is a chemical modification method, including the following steps:
[0054] Step 1: Preparation of HZ-TRAIL: Use the pET expression vector to clone the 114-281 gene sequence of the human TRAIL protein, and prepare the recombinant gene pET23dw-His with N-terminal histidine tag and isoleucine zipper motif - TRAIL recombinant protein of ILZ-hTRAIL114-281, referred to as HZ-TRAIL, such as figure 2 The schematic diagram showing the preparation of HZ-TRAIL;
[0055] Step 2: Extraction and purification of HZ-TRAIL: Transform the HZ-TRAIL prepared in Step 1 into Escherichia coli, and in LB culture medium containing 1mmol / L isopropylthiogalactopyranoside (IPTG) at 28°C , propagated for 8 hours, and then recombinantly expressed HZ-TRAIL in Escheri...
Embodiment 3
[0059] A trimeric TRAIL recombinant protein, such as figure 1 The schematic diagram of the preparation of PEG-HZ-TRAIL is shown, the inducing ligand is tumor necrosis factor-related apoptosis-inducing ligand TRAIL, and the preparation method is a chemical modification method, including the following steps:
[0060] Step 1: Preparation of HZ-TRAIL: Use the pET expression vector to clone the 114-281 gene sequence in the human TRAIL protein, and prepare a recombinant gene with an N-terminal histidine tag and an isoleucine zipper motif, pET23dw- The TRAIL recombinant protein of His-ILZ-hTRAIL114-281, referred to as HZ-TRAIL, such as figure 2 The schematic diagram showing the preparation of HZ-TRAIL;
[0061] Step 2: Extraction and purification of HZ-TRAIL: Transform the HZ-TRAIL prepared in step 1 into Escherichia coli, and in LB culture medium containing 1mmol / L isopropylthiogalactopyranoside (IPTG) at 30°C , multiplied for 6 hours, and then recombinantly expressed HZ-TRAIL in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com