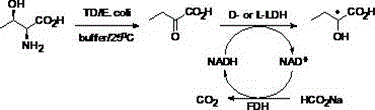
Novel biological preparation method of optically pure 2-hydroxy butyric acid
A technology of hydroxybutyric acid and a new method, applied in biochemical equipment and methods, using vectors to introduce foreign genetic material, lyase, etc., can solve the problems of high production cost, low optical purity, and low product concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of the cultivation of implementation example 1 genetically engineered bacterium and resting cell
[0029] Pick a single colony on the plate and inoculate it into 5ml of fermentation medium containing corresponding antibiotics, cultivate it for about 15 hours as a seed solution, inoculate it into 600ml of fermentation medium according to the inoculation amount of 1%, and cultivate it on a shaker at 37°C and 200rpm to OD 600 =0.6~0.8, add IPTG with a final concentration of 0.1mM to induce at 25°C for more than 10h, and collect the bacteria by centrifuging the culture medium at 8000rpm.
Embodiment 2
[0030]Implementation example 2 Threonine dehydratase, formate dehydrogenase, D-lactate dehydrogenase recombinant bacteria resting cells one-pot synthesis of (R)-2-hydroxybutyric acid
[0031] In a 250mL three-neck flask, add 89mL water, 8.93g threonine, 3.58g disodium hydrogen phosphate, 15.6g sodium formate, 1mL10mMPLP and 50mgNAD in sequence + , adjust the pH to 7.0, and then add 0.6g of resting cells of threonine dehydratase engineering bacteria (wet weight, 240U), 10mL of formate dehydrogenase enzyme solution (10U) and 1.6g of D-lactate dehydrogenase engineering bacteria of static For living cells (wet weight, 1493U), use the pH online control system to add 2M HCl solution to the reaction system, control the pH of the reaction system to maintain around pH 7.0, and control the reaction temperature at 25-30°C. After 24 hours of reaction, high performance liquid chromatography showed that the reaction was complete (Agilent1200seriesHPLC, Acclaim120C18column (4.6×250mm, 5.0μm)...
Embodiment 3
[0032] Implementation Example 3 Threonine Dehydratase, Formate Dehydrogenase, L-Lactate Dehydrogenase Recombinant Bacteria Static Cells Catalyze Threonine One-Pot Synthesis of (S)-2-Hydroxybutyric Acid
[0033] Replace the resting cells of D-lactate dehydrogenase engineering bacteria in Example 2 with resting cells of 1.6gL-lactate dehydrogenase engineering bacteria (wet weight, 133U), and react for 36 hours to obtain light yellow oil The product (S)-2-hydroxybutyric acid was 7.57g, the yield was 97%, and the ee value was greater than 99%. 1 HNMR (400MHz, CDCl 3 ):δ=4.26(dd,J=6.7,4.5Hz,1H),1.84-1.97(m,1H),1.76(dquin,J=14.3,7.2,7.2,7.2,7.2Hz,1H),1.02(t ,J=7.5Hz,3H). 13 CNMR (100MHz, CDCl 3 ):δ=179.59,71.31,27.27,8.95.MS(ESI):m / z:calcdforC4H7O3 - :103.0395[M-H] - ,found: 103.0405.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com