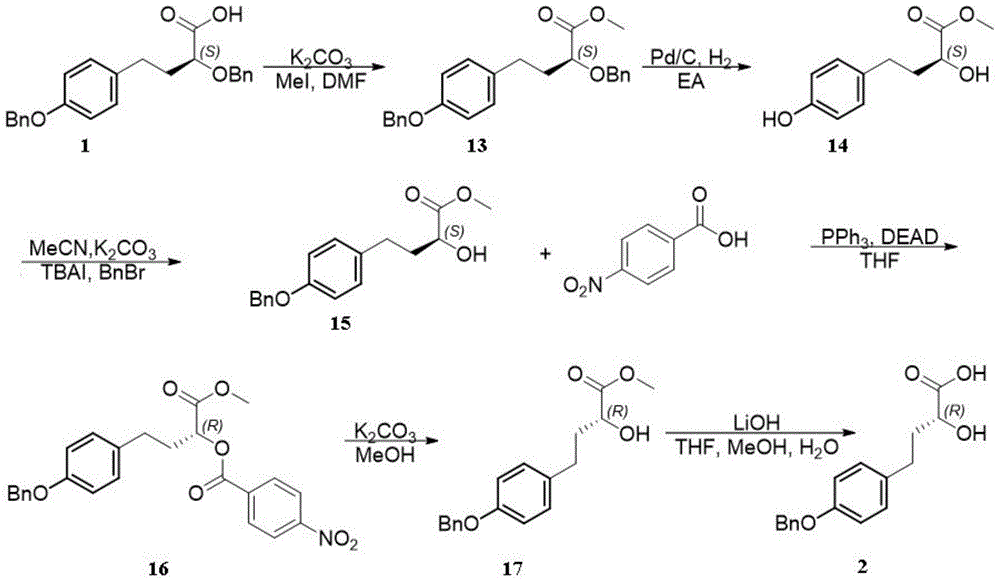
New synthesis method of two enantiomers of 4-(4-(benzyloxy)phenyl)-2-hydroxybutyric acid
A technology of enantiomers and benzyloxy, which is applied in the field of synthesis of chiral compounds, can solve problems such as no synthetic research, and achieve the effect of convenient scientific research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
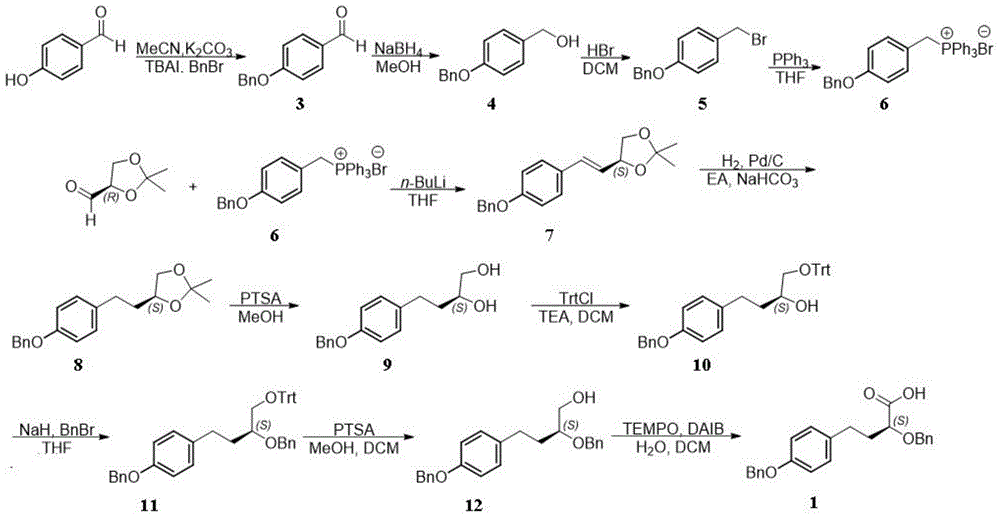
[0031] The synthetic method of (S)-4-(4-(benzyloxy) phenyl)-2-benzyl hydroxybutyric acid specifically comprises the steps:
[0032] 1) Synthesis of compound 3
[0033] Dissolve p-hydroxybenzaldehyde (10.0g, 81.88mmol) in acetonitrile (300mL), cool to zero, add potassium carbonate (16.98g, 122.83mmol), stir for ten minutes, add benzyl bromide (9.92mL, 83.52mmol) and Tetrabutylammonium iodide (3.0 g, 8.19 mmol), raised to room temperature, stirred overnight. After the reaction was complete, a saturated solution of ammonium chloride was added to quench the reaction. Extracted with ethyl acetate (300 mL), the organic phase was washed with saturated brine (50 mL), dried over anhydrous sodium sulfate, and the organic solvent was removed by rotary evaporation to obtain compound 3.
[0034] 2) Synthesis of Compound 4
[0035] Compound 3 was dissolved in methanol (200 mL), cooled to zero degree, sodium borohydride (6.05 g, 160 mmol) was slowly added, and kept stirring at zero degree...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com