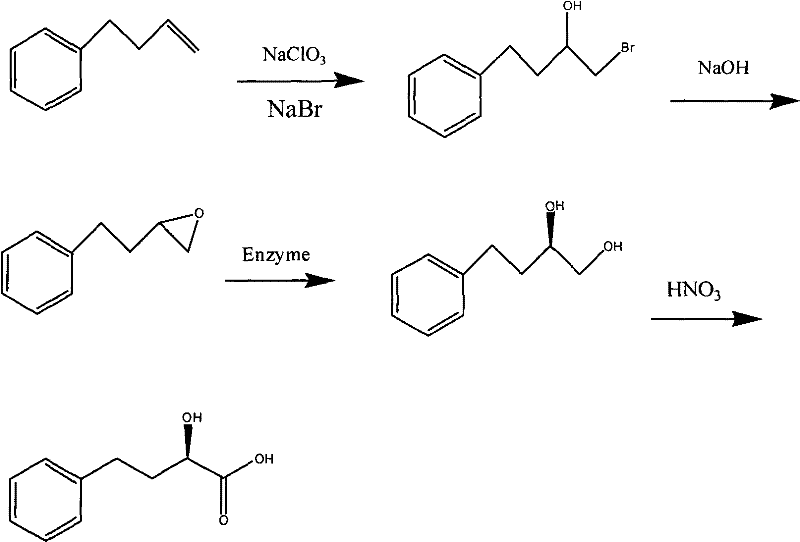
Preparation of (R) -4-phenyl-2-hydroxybutyrate
A technology of hydroxybutyric acid and phenyl, which is applied in the field of preparation of -4-phenyl-2-hydroxybutyric acid, can solve the problem of unsuitable resolution of 4-phenyl-2-hydroxybutyric acid and the price of D-malic acid. Expensive, the optical purity is only 18%, etc., to achieve the effect of good industrialization prospects, low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) 66g (0.5mol) of 4-phenyl-1-butene, sodium chlorate (NaClO 3 ) 26.6g (0.5mol), 100g of water into the reaction flask, stirred, heated to 85 ~ 90 ℃, then dropwise added 51.4g of sodium bromide (0.5mol), concentrated sulfuric acid 25ml, water 200ml of the mixed solution. The dropwise addition was completed in 3.5 hours. The reaction was continued for 1.5 hours, cooled naturally to room temperature, poured into a separatory funnel, and the oily substance in the lower layer was separated to obtain about 110 g of crude product 4-phenyl-1-bromo-2-butanol. The product was directly used in the lower reaction without purification.
[0024] (2) 110 g of the crude 4-phenyl-1-bromo-2-butanol obtained above was directly added to 120 g of 20% caustic soda aqueous solution, stirred and reacted at 30-35°C for 4 hours, cooled to room temperature, and left to stand 30 minutes for layering. Separate the upper product. Purified by high vacuum distillation to obtain 63 grams of 4-phe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com