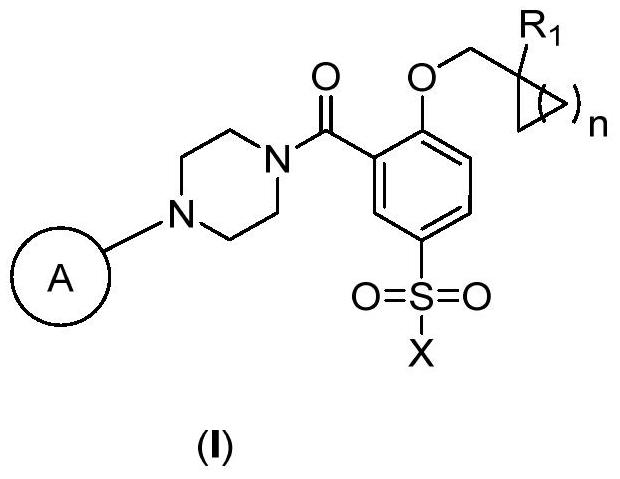
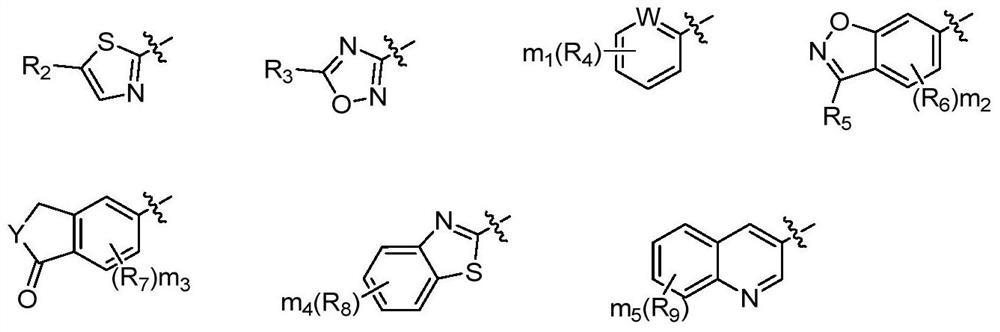
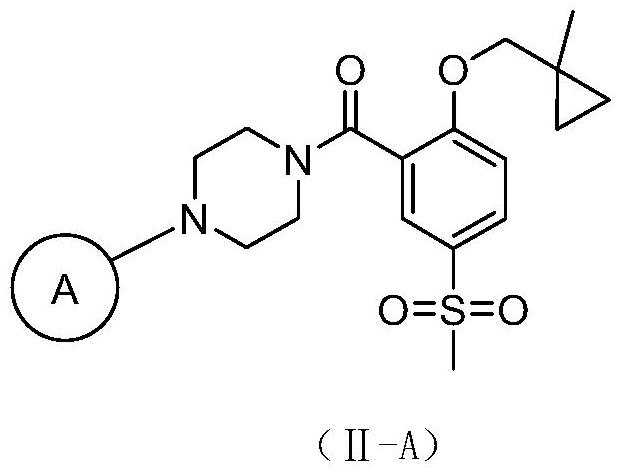
2-Substituted oxy-5-methylsulfonyl phenylpiperazinamide analogues and their preparation method and use
A kind of arylpiperazinamide and analog technology, applied in the field of 2-substituted oxy-5-methylsulfonyl phenylpiperazinamide analog and preparation thereof, can solve negative symptoms and cognitive symptoms without improvement And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] 4-(4-(2-((1-methylcyclopropyl)methoxy)-5-(methylsulfonyl)benzoyl)piperazin-1-yl)benzonitrile
[0158]
[0159] The first step: 5-(chlorosulfonyl)-2-fluorobenzoic acid
[0160] Take a 250mL three-necked flask, add chlorosulfonic acid (70mL, 1.06mol) and a stirrer, cool to 0°C, add 2-fluorobenzoic acid (25g, 179mmol) in batches, after the addition is completed, gradually rise to room temperature and react for 1h, 70 Stir overnight at ℃, cool to room temperature, add 500 mL of ice water dropwise, precipitate a solid, filter, wash the solid with water, and dry to obtain a white solid, which is directly carried out to the next reaction.
[0161] The second step: 2-fluoro-5-sulfinic acid
[0162] Dissolve sodium sulfite (125g, 0.99mol) in 400mL water, add 5-(chlorosulfonyl)-2-fluorobenzoic acid prepared in the first step in batches, stir at room temperature for 2h, cool to 0°C, add 20% sulfuric acid dropwise (150 mL), adjust the pH to 2, distill off the water, add 600 mL...
Embodiment 2
[0174] {4-[3-fluoro-5-(trifluoromethyl)pyridin-2-yl]piperazin-1-yl}{2-[(1-methylcyclopropyl)methoxy]-5-( Methylsulfonyl)phenyl}methanone
[0175]
[0176] The first step: 2-[(1-methylcyclopropyl)methoxy]-5-(methylsulfonyl)benzoic acid
[0177] Dissolve 2-fluoro-5-(methylsulfonyl)benzoic acid (436mg, 2mmol) in dimethylacetamide (5mL), add (1-methylcyclopropyl)methanol (344mg, 4mmol) and anhydrous Potassium carbonate (553 mg, 4 mmol). Rise to 150°C to react overnight. Evaporate the solvent, add water to dissolve, then adjust the pH value to about 2 with 1N hydrochloric acid, a white solid precipitates, filter the solid, and wash with water three times to obtain 2-[(1-methylcyclopropyl)methoxy]- 5-(methylsulfonyl)benzoic acid (460mg, white solid), the yield was 81%.
[0178] The second step: {4-[3-fluoro-5-(trifluoromethyl)pyridin-2-yl]piperazin-1-yl}{2-[(1-methylcyclopropyl)methoxy] -5-(methylsulfonyl)phenyl}methanone
[0179] 2-[(1-Methylcyclopropyl)methoxy]-5-(methyls...
Embodiment 3
[0182] (4-(2-fluoro-4-nitrophenyl)piperazin-1-yl)(2-((1-methylcyclopropyl)methoxy)-5-(methylsulfonyl)phenyl) ketone
[0183]
[0184] Take a 50mL single-necked bottle, add 2-((1-methylcyclopropyl)methoxy)-5-(methylsulfonyl)benzoic acid (81mg, 0.36mmol), 1-(2-fluoro-4-nitro phenyl)piperazine (80mg, 0.3mmol), HATU (171mg, 0.45mmol), TEA (0.16mL, 1.08mmol), DMF (10mL), react overnight at room temperature, add saturated sodium chloride (20mL), and wash with acetic acid Ethyl ester (20mL*3) was extracted, the organic phase was dried with anhydrous sodium sulfate, and concentrated to dryness to obtain a crude product, which was prepared by reverse phase to obtain 85 mg of a pure product, yield: 48%.
[0185] 1 H NMR (400MHz, CDCl 3 )δ8.02(dd,J=8.9,1.8Hz,1H),7.99–7.91(m,2H),7.89(d,J=2.3Hz,1H),7.00(d,J=8.8Hz,1H), 6.94(t,J=8.7Hz,1H),4.10–3.92(m,3H),3.80(d,J=9.1Hz,1H),3.62–3.24(m,6H),3.05(s,3H),1.21 (s, 3H), 0.49 (ddd, J = 16.0, 11.5, 6.0Hz, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com