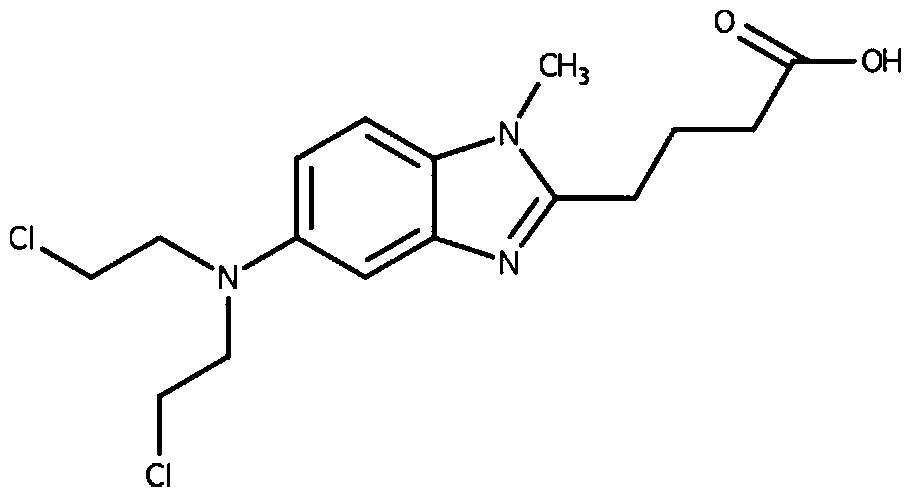
Medicinal composition and application of bendamustine
A technology of bendamustine and bendamustine hydrochloride, which is applied in the field of pharmaceutical compositions for tumor diseases and autoimmune diseases, can solve the problems of physical damage to patients, time-consuming, increased failure and impurities, etc., and achieve reduction Injection volume, shortening infusion time, and avoiding the effect of curative effect reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024]
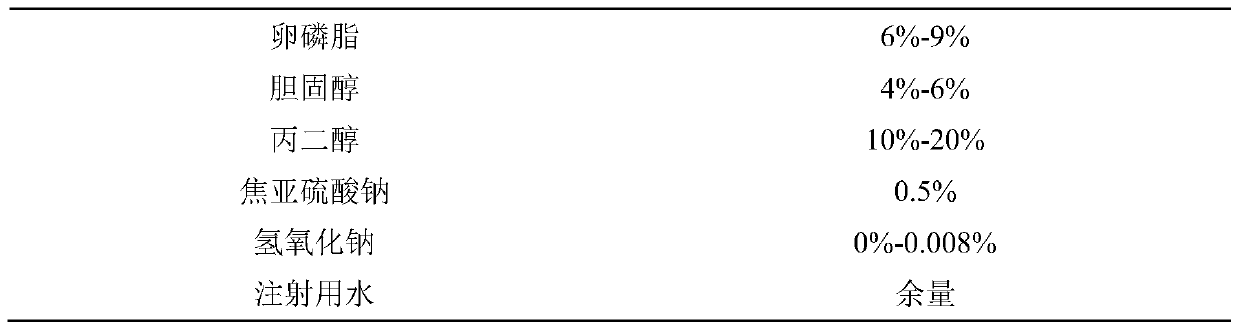
[0025] Take 6%-9% lecithin and 4%-6% cholesterol in a round bottom flask, reverse evaporation, add 2%-3% bendamustine hydrochloride in 10%-20% propylene glycol solution, mix well, add 0.5% sodium metabisulfite, add water for injection to a sufficient amount, adjust the pH to 2-3, the concentration of bendamustine hydrochloride is 25mg / mL, add activated carbon, stir, filter through a 0.45μm membrane to remove carbon, and filter through a 0.22μm membrane Sterilize, pack and serve.
Embodiment 2
[0027]
[0028] Take 6%-9% lecithin and 4%-6% cholesterol in a round bottom flask, reverse evaporation, add 2%-3% bendamustine hydrochloride in 5%-10% glycerol solution, mix well, add 0.5% sodium metabisulfite, add water for injection to a sufficient amount, adjust the pH to 2-3, the concentration of bendamustine hydrochloride is 25mg / mL, add activated carbon, stir, filter through a 0.45μm membrane to remove carbon, and filter through a 0.22μm membrane Sterilize, pack and serve.
Embodiment 3
[0030]
[0031]
[0032] Take 6%-9% lecithin, 4%-6% cholesterol in a round bottom flask, reverse evaporation, add 2%-3% bendamustine hydrochloride in 10%-20% propylene glycol solution, 0.1%-0.5 % Thioglycerol, mix well, add different antioxidants in the table, add water for injection to a sufficient amount, adjust pH to 2-3, bendamustine hydrochloride concentration is 25mg / mL, add activated carbon, stir, 0.45 Filter through a μm membrane to remove carbon, filter through a 0.22 μm membrane to sterilize, and pack in aliquots.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com