Application of medicine composition containing folium artemisiae argyi to preparing medicine for treating irritable bowel syndrome
A technology for irritable bowel syndrome and composition, which is applied in the direction of drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, which can solve the problems of complex preparation process, long treatment time, and slow drug effect, and achieve the goal of production process Simple, stable efficacy, and simple ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
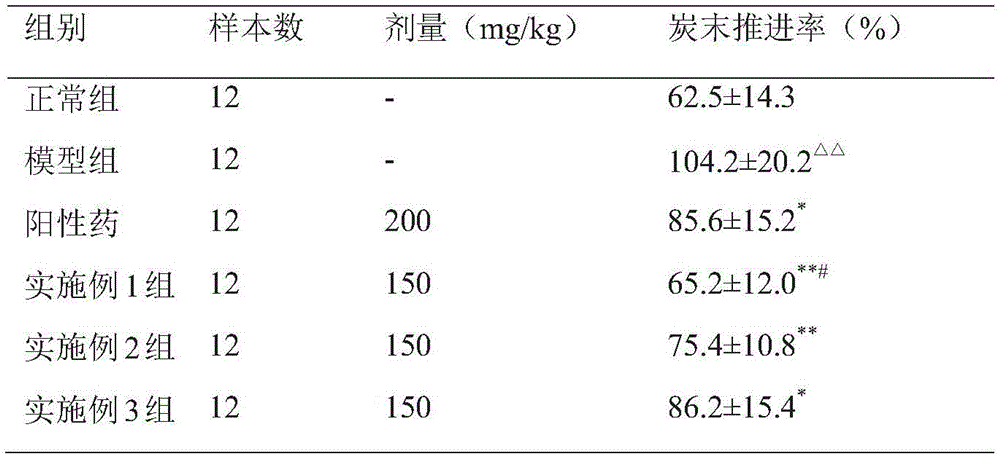
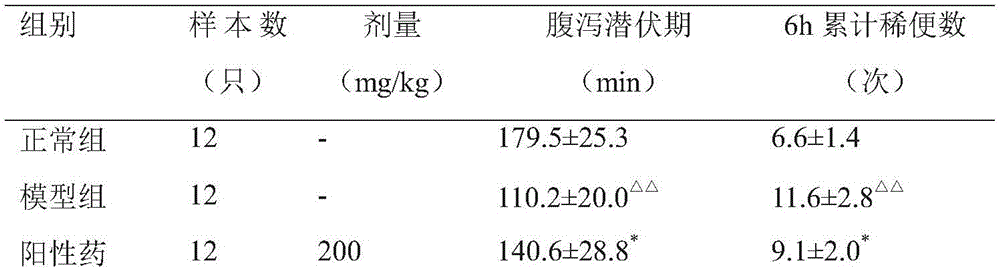
Embodiment 1
[0049] The pharmaceutical composition of Example 1 of the present invention includes the following preparation raw materials in parts by weight:
[0050] 13 parts of mugwort leaves, 20 parts of prickly pear, 11 parts of malt, 11 parts of treat servings and 6 servings of licorice.
[0051] The preparation method is as follows:
[0052] S1. Weigh each raw medicinal material, remove impurities, wash, dry, slice and pulverize into coarse powder;
[0053] S2, take the coarse powder of argyi leaves, place it in a supercritical carbon dioxide extraction device, add 45% of the total amount of coarse powder and 60% ethanol by volume, adjust the flow rate of carbon dioxide to be 15L / h, the extraction pressure is 20MPa, and the extraction temperature is 50°C, The extraction time was 2h, and the argyi leaf extract was obtained by separation under reduced pressure, and the argyi leaf dregs were retained;
[0054] S3. Take prickly pear, malt, phellodendron, astragalus, safflower, coix se...
Embodiment 2
[0059] The pharmaceutical composition of Example 2 of the present invention includes the following preparation raw materials in parts by weight:
[0060] 28 parts of mugwort leaves, 24 parts of prickly pear, 19 parts of malt, 17 parts of treat servings and 10 servings of licorice.
[0061] The preparation method is the same as in Example 1.
Embodiment 3
[0063] The pharmaceutical composition in Example 3 of the present invention includes the following preparation raw materials in parts by weight:
[0064] 21 parts of mugwort leaves, 22 parts of prickly pear, 15 parts of malt, 14 parts of treat servings and 13 servings of licorice.
[0065] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com