Preparation method of isavuconazole intermediate
A technology for isavuconazole and intermediates, which is applied in the field of preparation of isavuconazole intermediates, and can solve the problems of unsuitable for large-scale industrial production, complex preparation process, and high cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
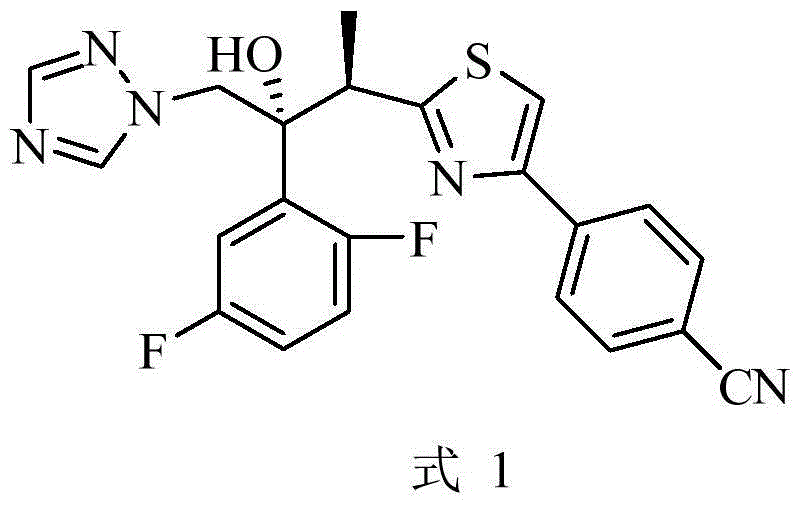
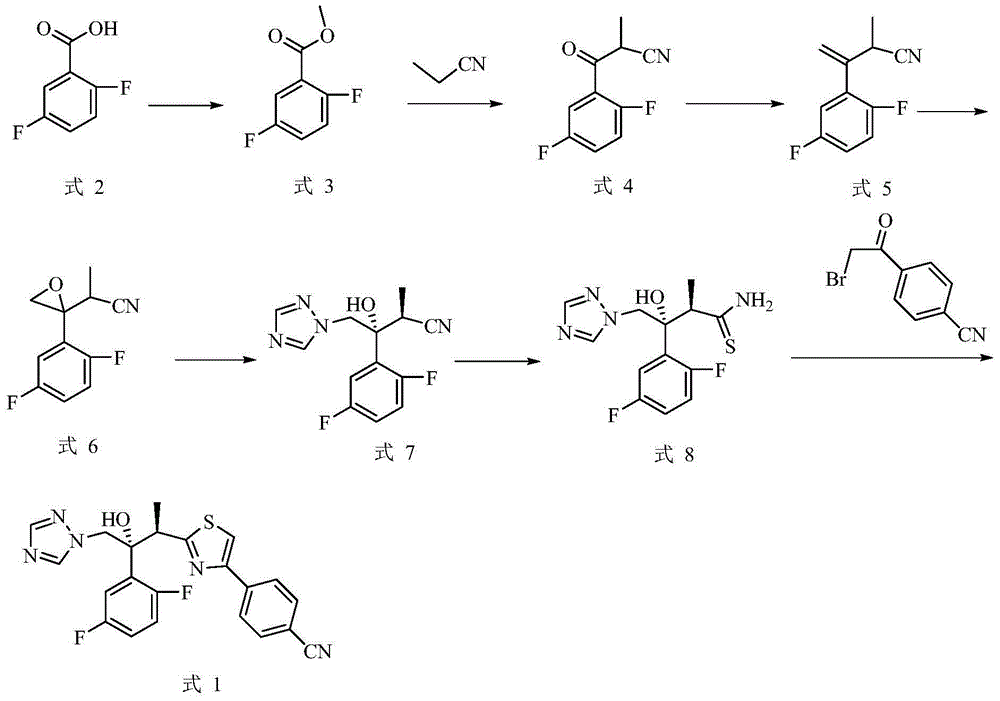
[0065] Example 4-(2-((2R,3R)-3-(2,5-difluorophenyl)-3-hydroxyl--4-(1H-1,2,4-triazol-1-yl ) butane -2-yl) thiazol-4-yl) preparation of benzonitrile (formula 1)
[0066]
[0067] (1) Preparation of 2,5-difluorobenzoic acid methyl ester (formula 3)
[0068] 2,5-difluorobenzoic acid (31.6g, 0.2mol) and thionyl chloride (35.7g, 0.3mol) were dissolved in 500mL of methanol, and the reaction was heated under reflux until the reaction was completed. After cooling, ice water was added, extracted with ethyl acetate, and the organic phase was dried and concentrated to obtain 31.2 g of product with a yield of 90.7%.
[0069] (2) Preparation of 3-(2,5-difluorophenyl)-2-methyl-3-oxopropionitrile (formula 4)
[0070] Dissolve methyl 2,5-difluorobenzoate (31.2g, 0.18mol), sodium ethoxide (14.7g, 0.22mol) and propionitrile (11.9g, 0.22mol) in 100mL of ethanol, heat the reaction to the end, and The solvent was concentrated to the remaining 20 mL, water was added, and extracted with ethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com