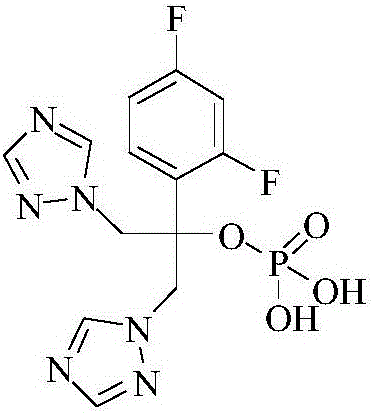
Method for preparing fosfluconazole
A technology for forsefluconazole and crude forsefluconazole is applied in the field of preparation of pharmaceutical compounds, and can solve the problems of high content of special impurities, affecting product quality, and low purity of forsefluconazole.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
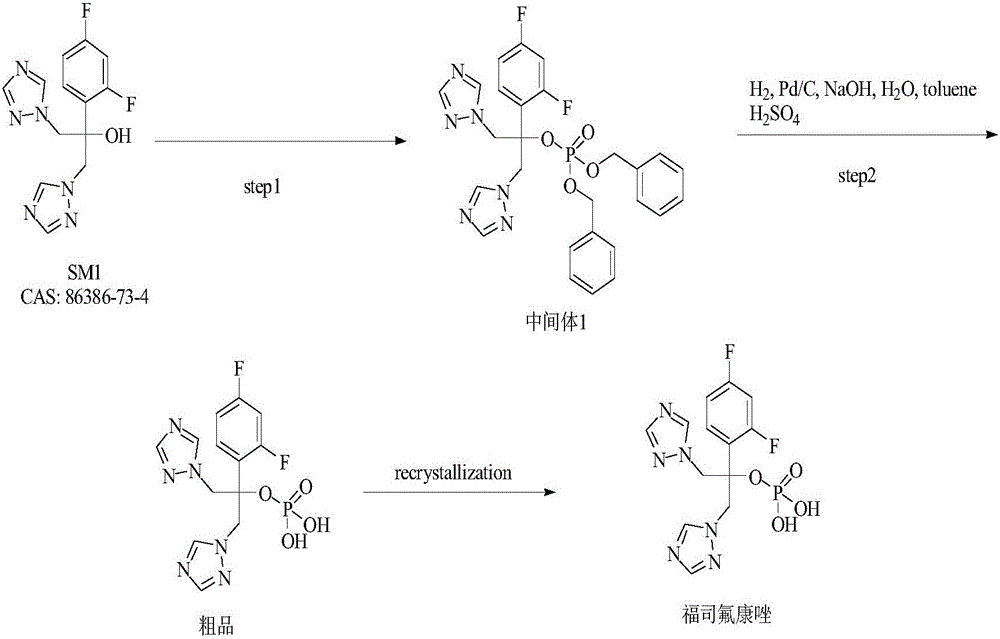
[0052] One, step one: the preparation of fosfluconazole intermediate (FSC-1)
[0053] 1) Ratio of materials
[0054]
[0055]
[0056] 2) Operation steps
[0057] Add 1.00kg of FSC-SM1 (fluconazole), 1.79kg of triethylamine, and 8L of dichloromethane into a 30L reaction kettle, stir and cool to -10~0°C;
[0058] Dissolve 538.33g of phosphorus trichloride into 2L of dichloromethane, add to the reaction kettle under stirring, control the rate of addition so that the temperature does not exceed 0°C;
[0059] After the dropwise addition, the temperature was raised to 10-20°C and stirred for 2 hours;
[0060] Drop 1.38kg of benzyl alcohol into the reaction bottle, control the rate of addition so that the temperature does not exceed 20°C; keep stirring at 10-20°C for 2 hours after dropping;
[0061] Cool the reaction solution to 0-10°C, drop 1.33kg of hydrogen peroxide into the reaction bottle, control the rate of addition so that the temperature does not exceed 20°C; keep ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com