A kind of synthetic method of bioadhesive thiol chitosan
A technology of thiolated chitosan and a synthesis method, which is applied in the field of biomedical materials, can solve the problems of reducing the catalytic activity of P-gp, and achieve the effects of ensuring performance, controllable reaction, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
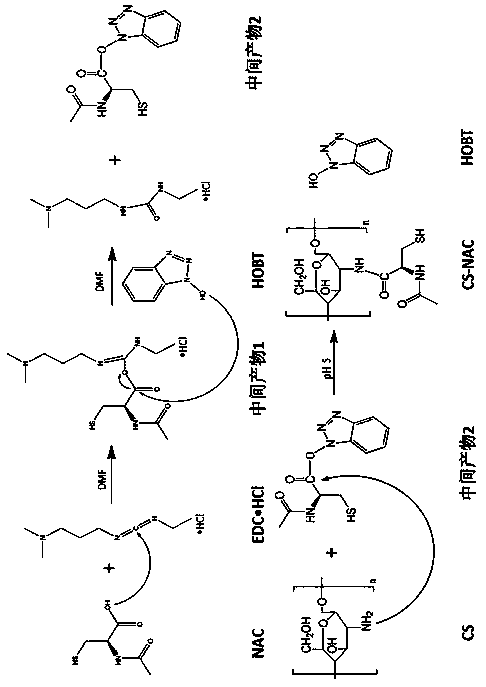
[0031] (1) Dissolve N-acetyl-L-cysteine (NAC), EDC·HCl, HOBT (molar ratio 4:1:1) with a total mass of 2g in 10mL N,N-dimethylformamide (DMF), continuous stirring at room temperature for 3-6 hours to activate NAC.
[0032] (2) Dissolve CS in a 20% hydrochloric acid solution to prepare a polymer solution with a concentration of 1.25% (w / v).
[0033] (3) Add activated NAC to the CS solution (the molar ratio of NAC to CS is 4:1), adjust the pH to 5 with NaOH, and react for 3-6 hours in the dark at room temperature.
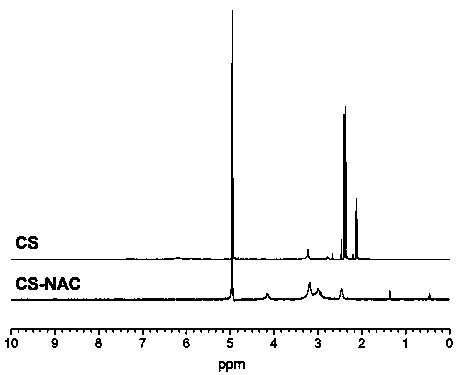
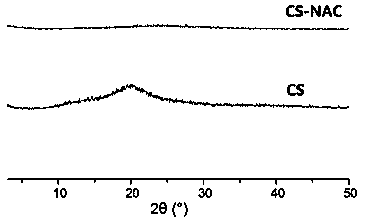
[0034] (4) To purify the product, remove the unreacted NAC. The reaction solution was dialyzed once with 5mmol / L hydrochloric acid solution, twice with 1% NaCl-containing hydrochloric acid (5mmol / L), and then with 5mmol / L The hydrochloric acid solution was dialyzed twice, each time was 12 hours, and all the dialysis processes were performed at 4°C and protected from light. Take out the solution after dialysis, pass through a 0.8μm filter membrane to remove impurities, an...
Embodiment 2
[0037] (1) Dissolve cysteine, EDC·HCl, HOBT (molar ratio 10:9:1) with a total mass of 2g in 10mL N,N-dimethylformamide (DMF), and stir continuously at room temperature 3-6 hours to activate cysteine.
[0038] (2) Dissolve CS in a 10% hydrochloric acid solution to prepare a polymer solution with a concentration of 0.8% (w / v).
[0039] (3) Add activated cysteine to the CS solution (the molar ratio of cysteine to CS is 20:1), adjust the pH to 4 with NaOH, and react for 3-6 hours in the dark at room temperature.
[0040] (4) To purify the product, to remove unreacted NAC, the reaction solution was dialyzed once with 1mmol / L hydrochloric acid solution, twice with 0.5% NaCl-containing hydrochloric acid (1mmol / L), and then with 1mmol / L The hydrochloric acid solution was dialyzed twice, each time was 12 hours, and all the dialysis processes were performed at 4°C and protected from light. Take out the solution after dialysis, pass through a 0.8μm filter membrane to remove impurities, an...
Embodiment 3
[0043] (1) Dissolve thioglycolic acid (TGA), EDC·HCl, HOBT (molar ratio 10:0.5:8) with a total mass of 2g in 10mL N,N-dimethylformamide (DMF), continuous at room temperature Stir for 3-6 hours to activate TGA.
[0044] (2) Dissolve CS in a 20% hydrochloric acid solution to prepare a polymer solution with a concentration of 1% (w / v).
[0045] (3) Add the activated TGA to the CS solution (the molar ratio of TGA to CS is 10:1), adjust the pH to 5 with NaOH, and react for 3-6 hours in the dark at room temperature.
[0046] (4) To purify the product, remove the unreacted TGA. The reaction solution was dialyzed once with hydrochloric acid solution containing 3mmol / L, twice with hydrochloric acid containing 1% NaCl (3mmol / L), and then with 3mmol / L The hydrochloric acid solution was dialyzed twice, each time was 12 hours, and all the dialysis processes were performed at 4°C and protected from light. Take out the solution after dialysis, pass through a 0.8μm filter membrane to remove impuri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com