Malposition lithium iron silicate and preparation method thereof
A technology of lithium iron silicate and silicate, which is applied in the field of high-performance lithium iron silicate cathode materials to achieve low ion migration activation energy, improve charge-discharge cycle performance, and increase reversible capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
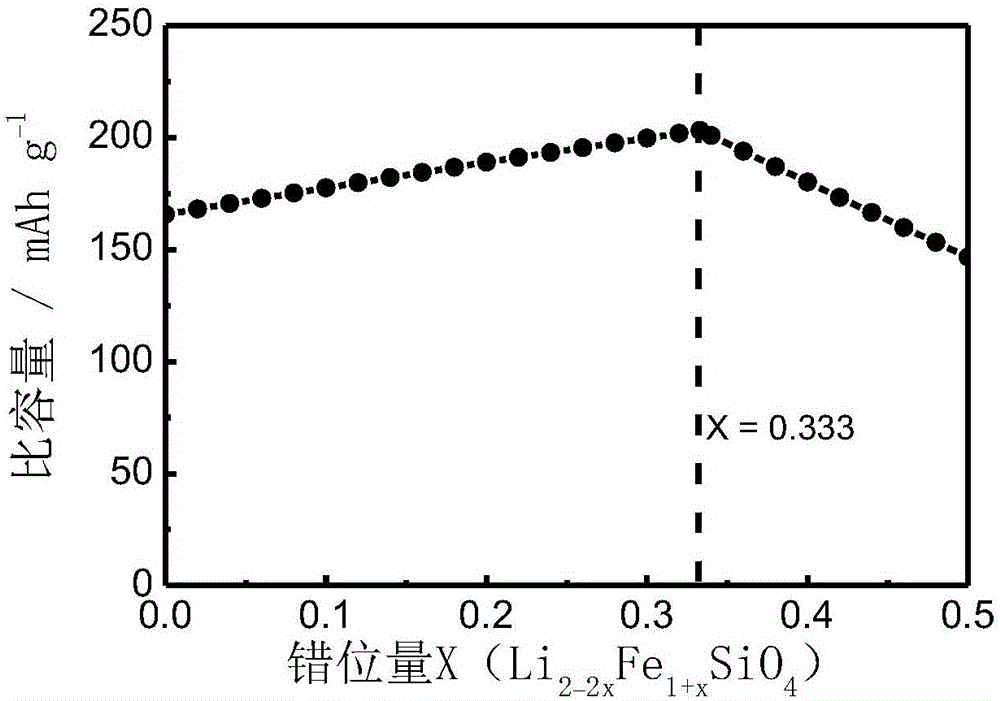
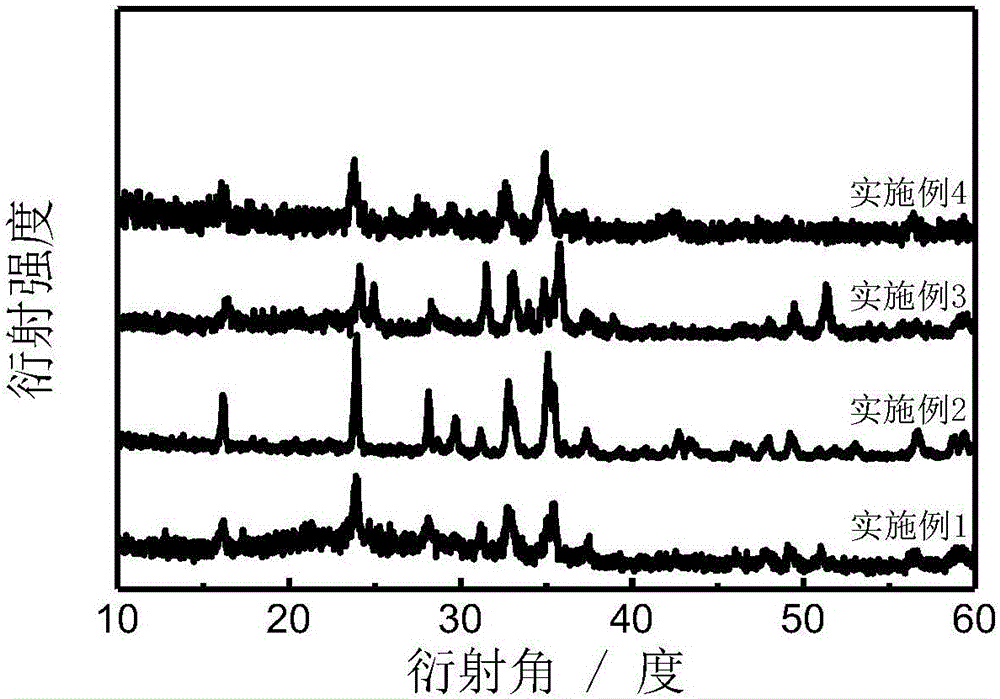
[0022] Example 1: Weigh lithium acetate, iron citrate and tetraethyl orthosilicate in deionized water at a molar ratio of 1.8:1.1:1, and add citric acid equal to the iron source as a complexing agent. Stir for about 12 hours to form a gel, then vacuum dry at 50°C for 12 hours, and continue aging at 90°C for 12 hours. Grinding and pulverizing the obtained xerogel, then pre-calcining in air at 300°C for 2 hours, and continuing to calcining at 650°C under nitrogen atmosphere for 6 hours, after cooling, Li 1.8 Fe 1.1 SiO 4 product. figure 2 The X-ray diffraction pattern of the obtained product is given, from which it can be seen that the obtained material basically maintains the original silicate structure; image 3 It is the electron micrograph of the obtained product, the material is a porous structure, and the particle size is about 50nm; Figure 4 It is the charge-discharge curve diagram of the obtained product, and the actual discharge capacity of the Li / Fe dislocation m...
Embodiment 2
[0023] Example 2: Weigh lithium acetate, ferric citrate and tetraethyl orthosilicate in deionized water at a molar ratio of 1.6:1.2:1, and add citric acid equal to the iron source as a complexing agent. Stir for about 12 hours to form a gel, then vacuum dry at 50°C for 12 hours, and continue aging at 90°C for 12 hours. Grinding and pulverizing the obtained xerogel, then pre-calcining in the air at 260°C for 1 hour, and continuing to calcining at 700°C for 12 hours under an inert nitrogen atmosphere, and obtaining Li 1.6 Fe 1.2 SiO 4 product. figure 2 The X-ray diffraction pattern of the obtained product is given, from which it can be seen that the obtained material basically maintains the original silicate structure; the actual discharge capacity of the Li / Fe dislocation material reaches 188mAhg -1 .
Embodiment 3
[0024] Example 3: Weigh lithium acetate, ferric citrate and tetraethyl orthosilicate in deionized water at a molar ratio of 1.4:1.3:1, and add tartaric acid equal to the iron source as a complexing agent, and stir at 60°C The gel was formed in about 12 hours, then vacuum dried at 60°C for 12 hours, and aged at 80°C for 24 hours. Grinding and pulverizing the obtained xerogel, then pre-calcining in air at 320°C for 1 hour, and continuing to calcining at 750°C in an inert nitrogen atmosphere for 6 hours, and then naturally cooling to obtain Li 1.4 Fe 1.3 SiO 4 product. figure 2 The X-ray diffraction pattern of the obtained product is given, from which it can be seen that the obtained material basically maintains the original silicate structure; the actual discharge capacity of the Li / Fe dislocation material reaches 192mAhg -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com