Modified bacterial collagen-like proteins
一种胶原蛋白、蛋白的技术,应用在修饰的细菌胶原蛋白样蛋白领域,能够解决复杂、增加系统复杂性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
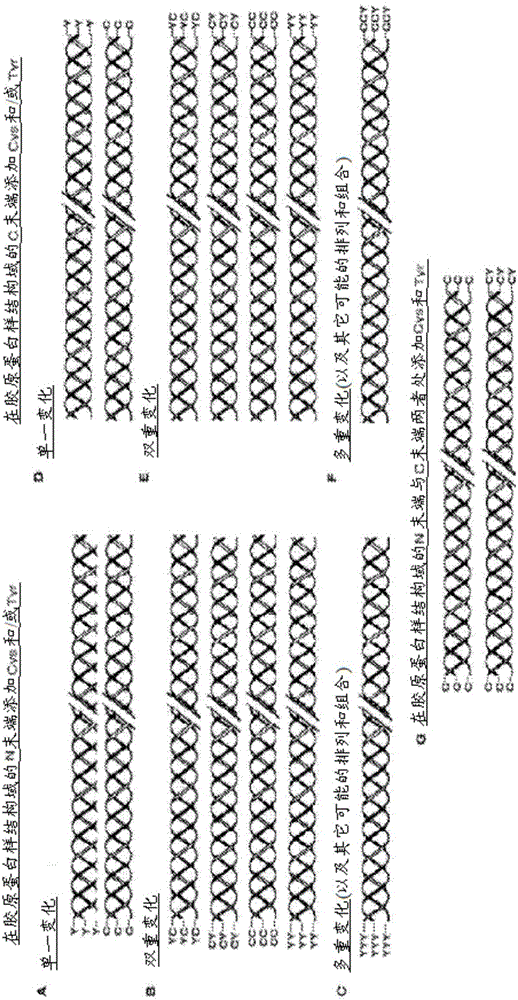
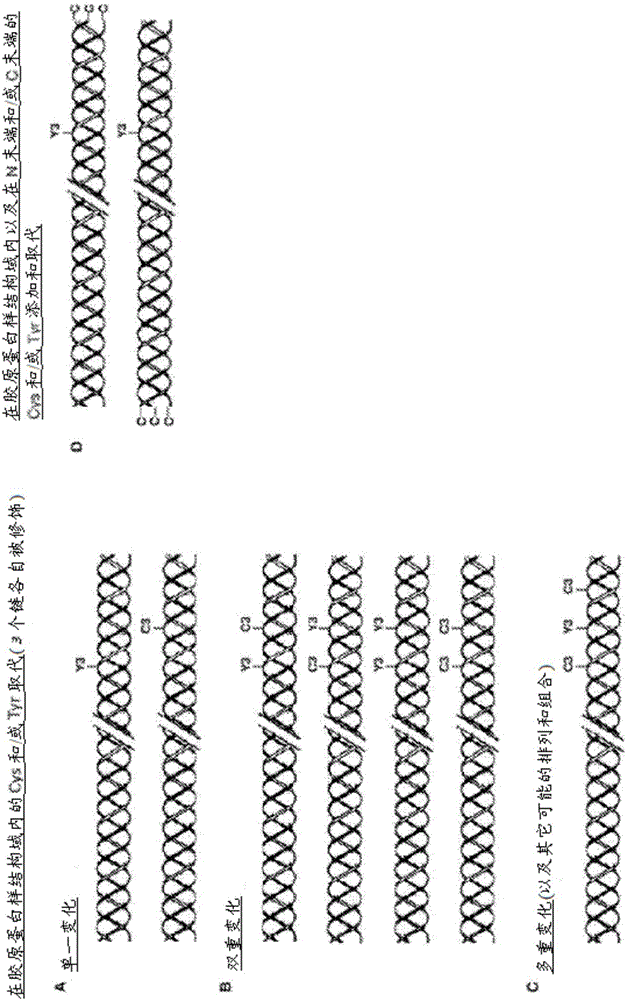
[0200] Example 1: Bacterial collagen sequences with Tyr residues introduced at the C-terminus
[0201] The combined globular and collagen-like portion of the scl2.28 allele (Q8RLX7) encoding the Scl2.28 protein, but lacking The DNA sequence of the fragment of the C-terminal linked domain is recorded as GenBank: AY069936.1. Introduce His into this sequence at the N-terminus 6 tag, and the thrombin / trypsin cleavage sequence LVPRGSP (SEQ ID NO: 1) was inserted between the N-terminal globular domain (V) and the following (Gly-Xaa-Yaa) n Between triple helix / collagen-like (CL) domain sequences. The triplet sequence GKY is included at the C-terminus of the CL domain, followed by a stop codon, with NdeI and BamHI cloning sites. DNA for this design was synthesized commercially without any codon optimization. SEQ ID NO: 2 is the final DNA construct. SEQ ID NO: 3 is the translated protein sequence.
Embodiment 2
[0202] Example 2: Bacterial collagen sequences with Cys residues introduced at the N-terminus
[0203] The DNA sequence of collagen containing triple helical repeats from Candidatus Solibacterusitatus Ellin 6076 was obtained as entry ABJ82342 from data provided in the National Institutes of Health, Bethesda, MD 20894, USA. The DNA sequence of the V domain from Rhodopseudomonas palustris was obtained as YP_001993084 from data provided in the National Institutes of Health, Bethesda, MD 20894, USA database. The protein sequence was translated into a nominal DNA sequence and a composite gene was designed maintaining the correct coding frame with the Met initiation signal followed by the additional amino acid sequence Gly-Cys-Pro containing Cys residues followed by the S The CL domain of .usitatus, followed by the V domain from Rhodopseudomonas palustris, and finally followed by the C-terminal His 6 tags and stop codons. Terminal restriction sites outside the coding sequence were...
Embodiment 3
[0204] Example 3: Bacterial collagen sequences with both Cys and Tyr residues introduced at the C-terminus
[0205] The sequence of this example was prepared as described in Example 1, except that the sequence at the C-terminus of the protein was Gly-Tyr-Cys at the end of the CL domain, followed by a stop codon and a restriction site, The DNA of this sequence was commercially synthesized without any codon optimization. The sequence of this construct is shown in SEQ ID NO:6 and 7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com