Application of human amniotic mesenchymal stem cells
A technology of mesenchymal stem cells and human amniotic membrane, applied in the field of biomedicine, can solve problems such as human amniotic mesenchymal stem cells that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Application of human amniotic mesenchymal stem cells in the preparation of preparations for treating myocardial ischemia-reperfusion injury during cardiopulmonary bypass. The specific application method is: suspending human amniotic mesenchymal stem cells in physiological saline to prepare human amniotic mesenchymal stem cell injection, and then injecting it intravenously.
Embodiment 2
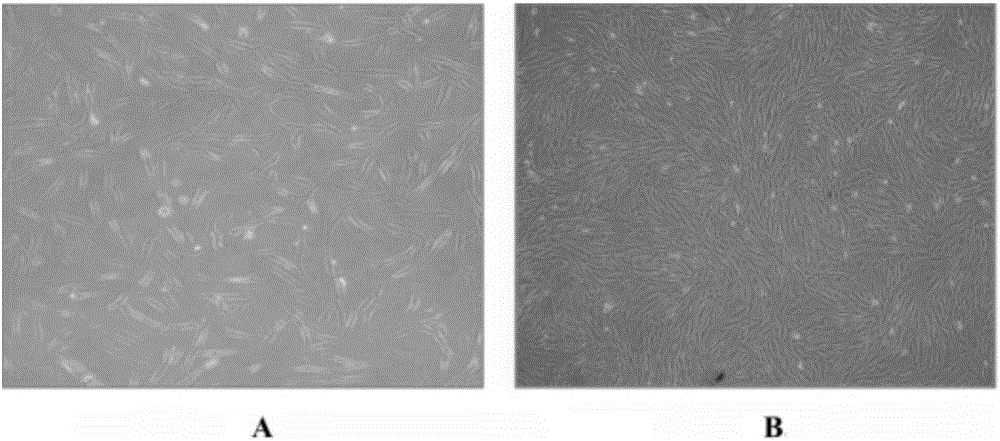
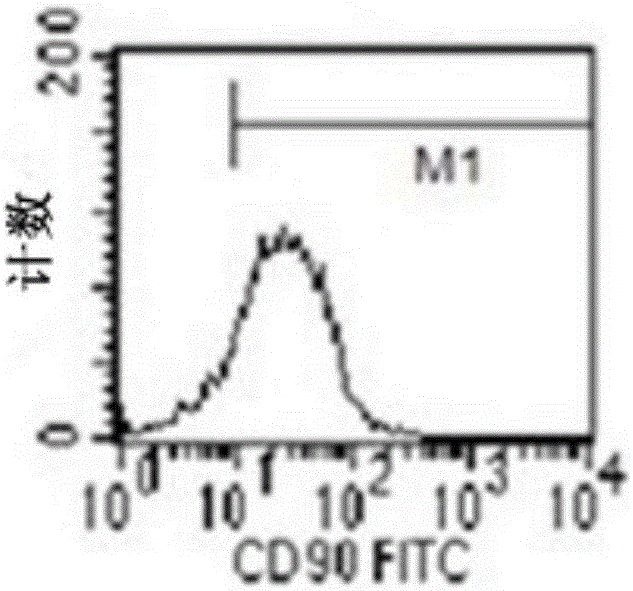
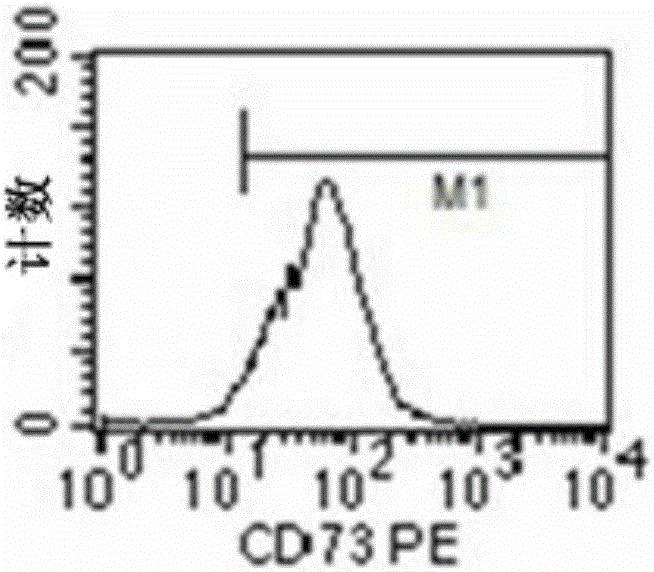
[0129] Application of human amniotic mesenchymal stem cells in the preparation of preparations for treating myocardial ischemia-reperfusion injury during cardiopulmonary bypass. The specific application method is: suspending human amniotic mesenchymal stem cells in physiological saline to prepare human amniotic mesenchymal stem cell injection, and then injecting it intravenously. Human amniotic mesenchymal stem cells are the 3rd to 5th passage cells obtained through subculture, and more than 90% of their cytoplasm express vimentin, and the expression of CD90, CD105, CD44 and CD73 is positive, and CD34, CD45, CD11b, CD19 and HLA-DR negative.
Embodiment 3
[0131] Application of human amniotic mesenchymal stem cells in the preparation of preparations for treating myocardial ischemia-reperfusion injury during cardiopulmonary bypass. The specific application method is: suspending human amniotic mesenchymal stem cells in physiological saline to prepare human amniotic mesenchymal stem cell injection, and then intravenously injecting it through the femoral vein. Human amniotic mesenchymal stem cells are cultured according to the method described in the invention patent No. 2011100809686. They are the 3rd to 5th generation human amniotic mesenchymal stem cells obtained through subculture, and more than 90% of their cytoplasm express vimentin, and Expression of CD90, CD105, CD44, and CD73 was positive, and CD34, CD45, CD11b, CD19, and HLA-DR were negative.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com