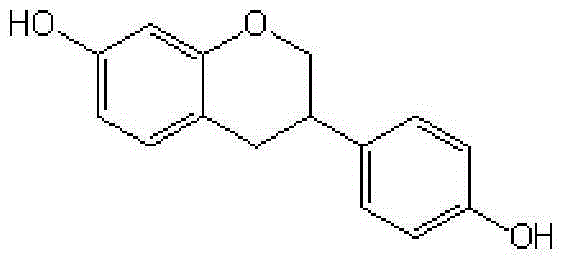
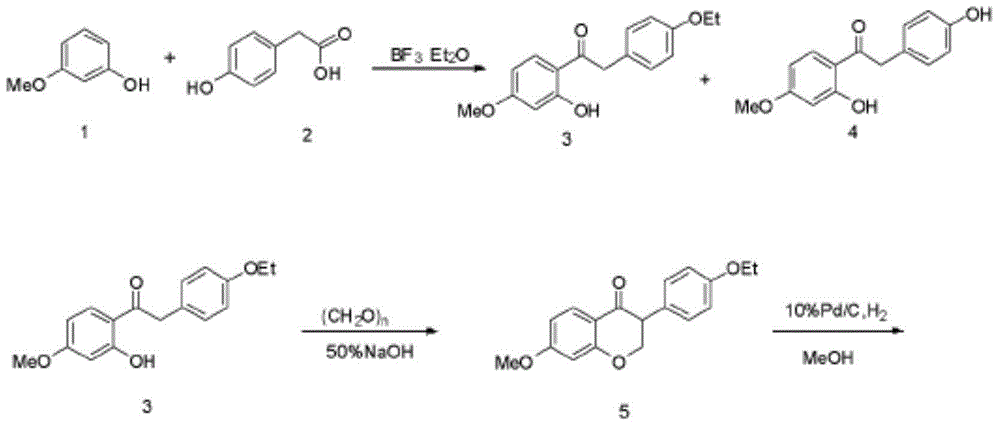
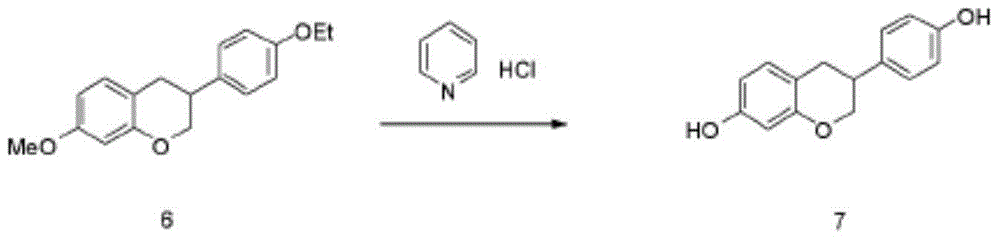
Synthetic method for equol
A synthesis method and equol technology are applied in the field of organic compound preparation process improvement, can solve problems such as being unsuitable for industrial scale-up production, increase production cost, and be expensive, and achieve the effects of short synthesis steps, reduced total cost, and low price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1) Preparation of compound (B)
[0038] Put 50g (0.1968mol) of daidzein, 300g of 1,2-dichloroethane, 95g (0.9314mol) of acetic anhydride and 12g of concentrated sulfuric acid into a washed and dried 1000ml reaction flask. After the injection is completed, the temperature is raised to 60°C for 4 hours. After the reaction is completed, 1,2-dichloroethane is recovered under reduced pressure. After the recovery is completed, the residue is added back to the ice-water mixture, and a large amount of crystals are precipitated. Filtered and dried to obtain a White solid 55g. That is compound (B). Yield: 82.66% (calculated based on compound A). Melting point: 187.2-187.4°C, ESI (M / Z): 338.
[0039] 2) Preparation of compound (C)
[0040] Put 6g of 5% Pd / C, 200g of N,N-dimethylformamide, 2g (0.0196mol) of acetic anhydride and 20g (0.0592mol) of compound (B) into a washed and dried 500ml autoclave, and seal the autoclave , start stirring, after nitrogen gas exchange, fill wit...
Embodiment 2
[0046] 1) Preparation of compound (B)
[0047] Put 50g (0.1968mol) of daidzein, 300g of 1,2-dichloroethane, 95g (0.9314mol) of acetic anhydride and 12g of concentrated sulfuric acid into a washed and dried 1000ml reaction flask. After the injection is completed, the temperature is raised to 85°C for 2 hours. After the reaction is completed, 1,2-dichloroethane is recovered under reduced pressure. After the recovery is completed, the residue is added back to the ice-water mixture, and a large number of crystals are precipitated. Filtered and dried to obtain a White solid 55.53g. That is compound (B). Yield: 83.46% (calculated as compound A). Melting point: 187.1-187.4°C, ESI (M / Z): 338.
[0048] 2) Preparation of compound (C)
[0049] Put 15g of 1% Pd / C, 240g of N,N-dimethylformamide, 2g (0.0196mol) of acetic anhydride and 20g (0.0592mol) of compound (B) into a washed and dried 500ml autoclave, and seal the autoclave , start stirring, after nitrogen gas exchange, fill with ...
Embodiment 3
[0055] 1) Preparation of compound (B)
[0056] Put 50g (0.1968mol) of daidzein, 300g of 1,2-dichloroethane, 95g (0.93213mol) of acetic anhydride and 12g of concentrated sulfuric acid into a washed and dried 1000ml reaction flask. After the injection, the temperature was raised to 75°C for 3 hours. After the reaction was completed, 1,2-dichloroethane was recovered under reduced pressure. After the recovery was completed, the residue was added back to the ice-water mixture, and a large amount of crystals were precipitated. Filtered and dried to obtain a White solid 55.98g. That is compound (B). Yield: 84.15% (calculated as compound A). Melting point: 187.1-187.3°C, ESI (M / Z): 338.
[0057] 2) Preparation of compound (C)
[0058] Put 2g of 10% Pd / C, 160g of N,N-dimethylformamide, 2g (0.0196mol) of acetic anhydride and 20g (0.0592mol) of compound (B) into a washed and dried 500ml autoclave, and seal the autoclave , start stirring, after nitrogen gas exchange, fill with H 2 T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com