(2-pyridylaldehyde)-2,6 pyridine diacylhydrazone copper compound, preparation method and application thereof
A technology of pyridine bisacylhydrazone copper and pyridine bisacylhydrazone, which is applied in the field of preparation of -2,6 pyridine bisacylhydrazone copper compound, and achieves the effects of high anticancer activity, simple preparation method and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
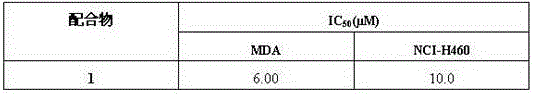
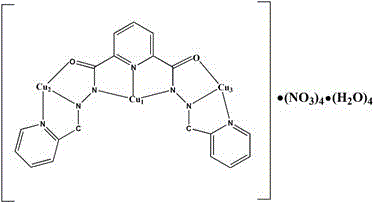
[0016] The steps are: 0.25mmol (2-pyridinecarbaldehyde)-2,6 pyridine bisacylhydrazone (H 2 L) (92.8mg) and 0.75mmolCu(NO 3 )·3H 2 O (181mg) was dissolved in 20mL of methanol, stirred at room temperature for 5h, filtered, and the filtrate was placed in 6 test tubes at an average of 3ml each, and diffused with ethanol and ether. After one week, dark green flaky crystals were obtained, and the yield of 6 test tubes was 64.93 mg, yield 28.4%. Melting point greater than 300 ° C. The highest yield of this compound was 68.6%.
[0017] The copper compound of the present invention is analyzed by X-single crystal diffraction, and the obtained crystallographic data are as follows: the compound belongs to the monoclinic system, the space group is P2(1) / n, and the unit cell parameters are: a=11.3200(9)?, b=13.2506(11)?, c=30.683(3)?, α=90°, β=92.639(2)°, γ=90°, V=3021.4(4)? 3 ,Z=4,D c =1.930Mg·m -3 ,μ=2.194mm -1 , F(000)=1756, 2.523 , the independent diffraction point is 5308, R1=0....
Embodiment 2
[0019] 0.2mmo(2-pyridinecarbaldehyde)-2,6pyridine bisacylhydrazone (H 2 L) and 0.6mmolCu(NO 3 )·3H 2 O mixed and dissolved in 15mL of methanol, stirred at room temperature for 5h, filtered, the filtrate evaporated, the filtrate was placed in 5 test tubes with an average of 3ml each, and ethanol and ether were added dropwise to diffuse. After 5 days, dark green flaky crystals were obtained, with a melting point greater than 300°C . The highest yield of this compound was 67.0%.
Embodiment 3
[0021] 0.15mmo(2-pyridinecarbaldehyde)-2,6pyridine bisacylhydrazone (H 2 L) and 0.45mmolCu(NO 3 )·3H 2 O mixed and dissolved in 15mL of methanol, stirred at room temperature for 8h, filtered, and the filtrate was volatilized, and the filtrate was placed in 5 test tubes with an average of 3ml each, and diffused by dropping ethanol and ether. After 4 days, dark green flaky crystals were obtained, with a melting point greater than 300°C . The highest yield of this compound was 66.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com