Detection reagent kit and method of heat-resistant microorganisms in elemene lipidosome injection semi-finished products
A technology of elemene lipid and testing method, which is applied in the fields of medical devices and testing methods to achieve the effects of saving manpower, improving testing efficiency and ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Elemene injection semi-finished heat-resistant microorganism test kit can be used 2-4 times.
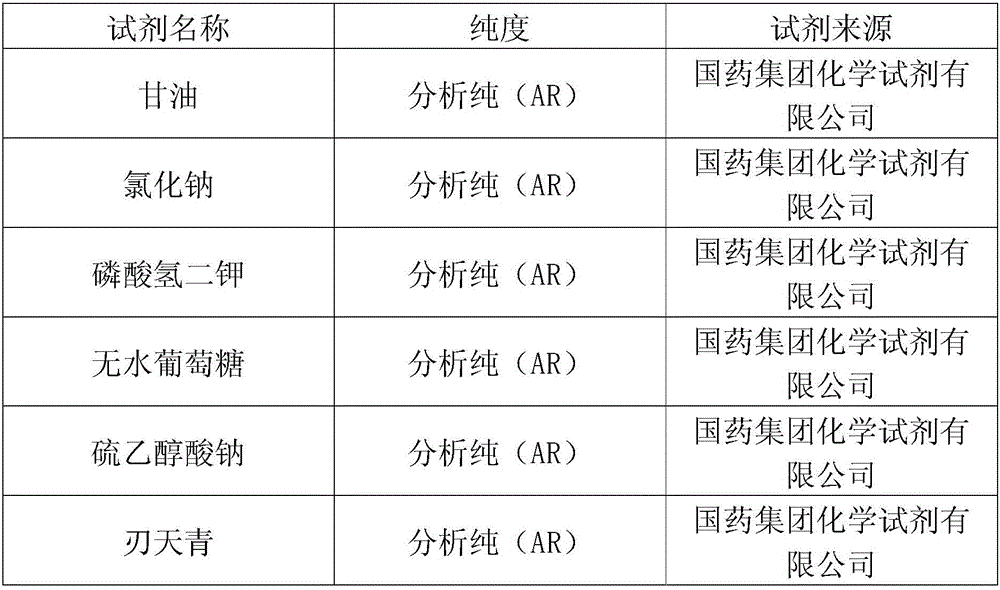
[0037] Kit composition: 1. Sterile elemene injection, 10ml×20 tubes;
[0038] 2. Thioglycollate fluid medium (powder), 50-80g;
[0039] 3. Tryptone soy agar medium (powder), 65-90g;
[0040] 4. Tryptone soy liquid medium (powder), 50-75g;
[0041] 5. 10%-20% (mL / mL) sterile glycerin solution, 40-60ml;
[0042] 6.10 6 cfu / ml biological indicator Bacillus stearothermophilus, 10-30ml.
[0043] Store the liquid at 2-8°C after opening, shelf life: 1 year.
[0044] Preparation Example
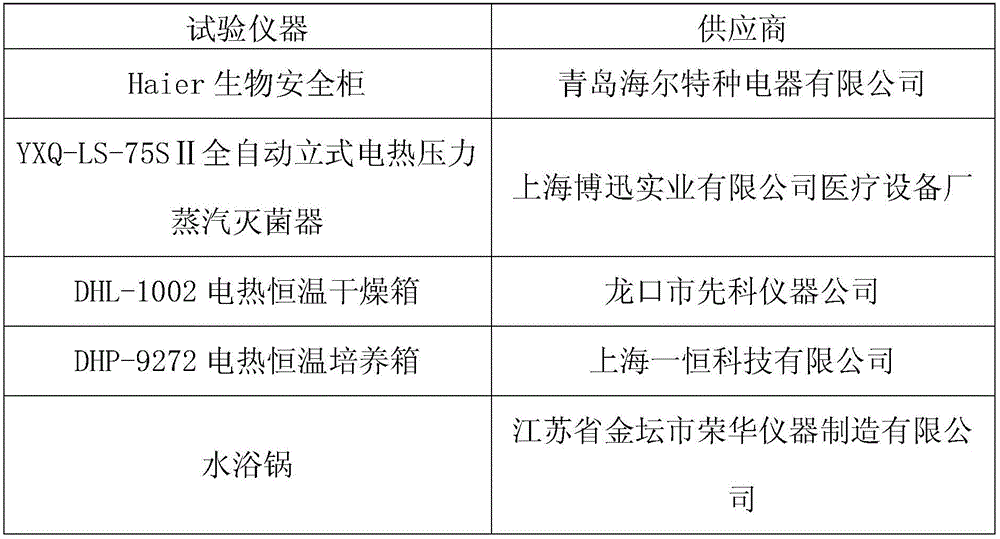
[0045] Preparation method of sterile elemene injection: take 200ml of elemene injection semi-finished product, pack into 10ml glass ampoules, and sterilize in YXQ-LS-75SⅡ automatic vertical electric pressure steam sterilizer at 121℃ for 15min.
[0046] The formula of thioglycolate fluid medium powder is: tryptone 28-38g; sodium chloride 4-7g; yeast extract powder 8-15g; anhydrous glu...
Embodiment 2
[0049]Example 2 can be detected by using the Elemene Injection Semi-finished Heat-resistant Microorganism Testing Kit described in Example 1.
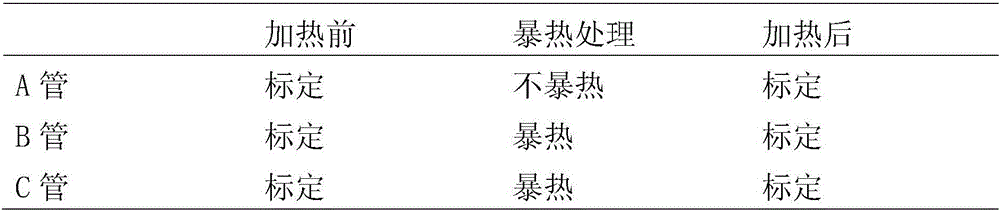
[0050] 1. Boiling test
[0051] 1.1 Take one sample each before, during and after potting, and use membrane filtration to trap microorganisms on a 0.45 μm filter membrane;
[0052] 1.2 Transfer the filter membrane after filtration and rinsing to a capped test tube containing 10ml sterile elemene injection;
[0053] 1.3 Place the test tube in a boiling water bath for heat treatment. When the test tube is immersed in the boiling water bath, start timing for about 1-4 minutes. After 15-30 minutes, quickly place the test tube in cold water to cool to room temperature;
[0054] 1.4 Inject about 50-100mL of thioglycolate fluid medium into the test tube;
[0055] 1.5 Place the test tube at 30-35 ° C for 48-72 hours, transfer 1-2 mL to another sterile capped empty test tube, inject about 50-100 mL of thioglycolate fluid medium, and replace t...
Embodiment 3
[0077] Embodiment 3 application kit of the present invention and the sterility detection result that detection method obtains are shown in the table below:
[0078] The specific implementation in 2015
[0079]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com