Novel dihydro pteridinone derivative, preparing method thereof and application to medicine
A technology of dihydropteridone and derivatives is applied in the directions of drug combination, antitumor drug, organic chemistry, etc., can solve the problem of no long-acting drug and the like, achieves reduction of toxic side effects and adverse reactions, simple preparation method, The effect of increasing selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
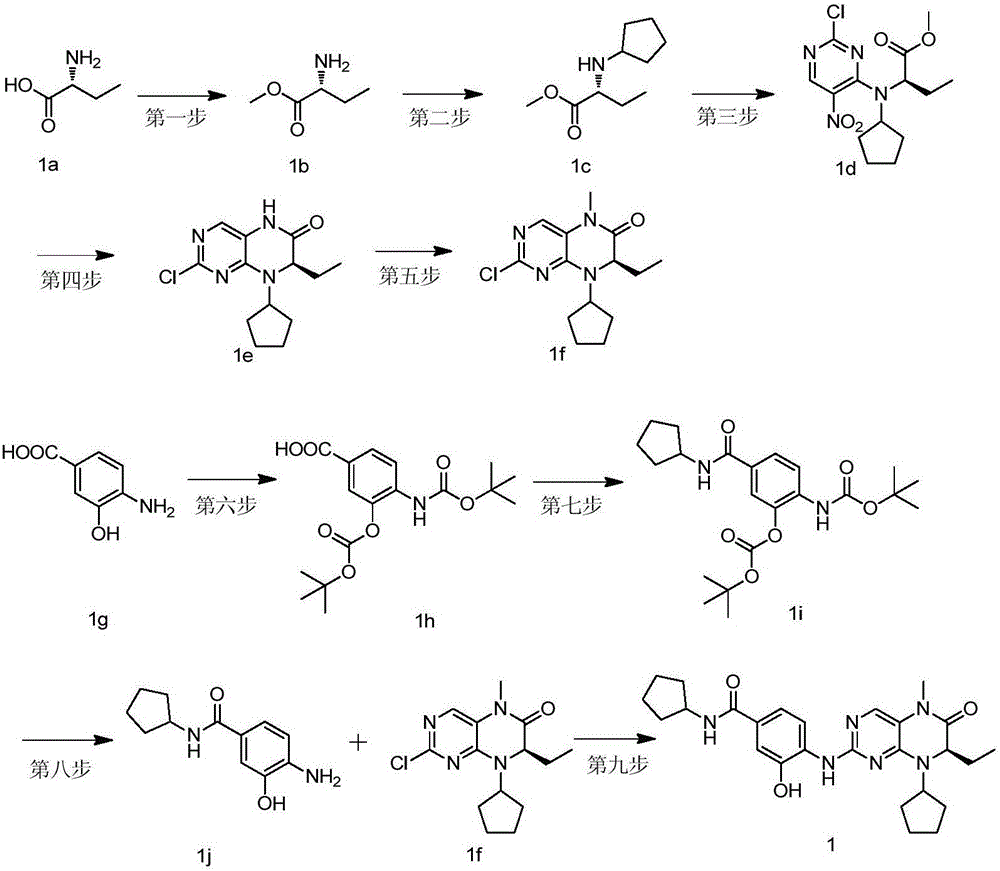
[0027] Example 1, (R)-N-cyclopentyl-4-(8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,7,8-tetrahydro-pterene Pyridin-2-ylamino)-3-hydroxyl-benzamide (compound 1), the reaction scheme is as follows figure 1 Shown:
[0028] The first step, (R)-2-amino-butyric acid methyl ester (compound 1b)
[0029] Under ice bath, take (R)-2-amino-butyric acid (compound 1a) (10g, 96.9mmol) and dissolve it in 120mL of methanol, slowly add thionyl chloride (16mL, 0.22mol) dropwise, reflux and stir for 2 hours , cooled to room temperature, concentrated the reaction solution under reduced pressure to obtain a light yellow oil, then added 100mL tert-butyl methyl ether and stirred for 0.5 hours, a white solid was precipitated, filtered by suction, and dried in vacuo overnight to obtain compound 1b (10.5g, white solid, yield 93%). 1 HNMR (600MHz, CDCl3) δ8.78(s, 2H), 4.15(d, J=5.2Hz, 1H), 3.82(s, 3H), 2.14(q, J=7.4Hz, 2H), 1.12(t, J=7.5Hz, 3H).
[0030] The second step, (R)-2-cyclopentyl-amino-butyric ...
Embodiment 2
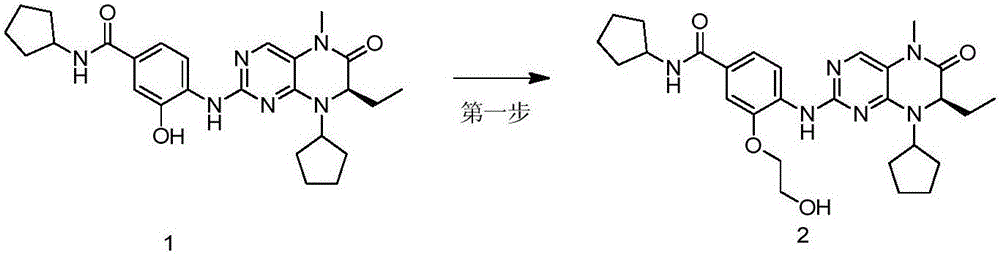
[0046] Example 2, (R)-N-cyclopentyl-4-(8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,7,8-tetrahydro-pterene Pyridin-2-ylamino)-3-(2-hydroxyethoxy)benzamide (Compound 2)
[0047] Such as figure 2 As shown, compound 1 (100mg, 0.209mmol), 2-bromoethanol (26.7μl, 0.376mmol) and potassium carbonate (57.8mg, 0.418mmol) were dissolved in acetone, reacted at 60°C for 12 hours, and cooled to At room temperature, the reaction solution was concentrated under reduced pressure, distilled water was added, extracted with ethyl acetate (50mL×3), washed with saturated brine, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure by column chromatography (petroleum Ether: ethyl acetate = 1:2) separation and purification to obtain the target product (55 mg, light yellow solid, yield 47.6%). 1 HNMR (600MHz, CDCl 3 )δ8.39(d, J=8.5Hz, 1H), 7.54(s, 1H), 7.40(d, J=1.1Hz, 1H), 7.26(dd, J=8.5, 1.7Hz, 1H), 6.19( d,J=6.8Hz...
Embodiment 3
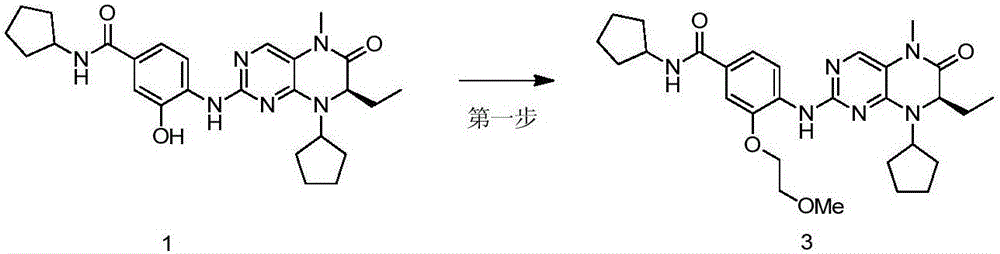
[0048] Example 3, (R)-N-cyclopentyl-4-(8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,7,8-tetrahydro-pterene Pyridin-2-ylamino)-3-(2-methoxyethoxy)benzamide (Compound 3)
[0049] Such as image 3 , Dissolve compound 1 (100mg, 0.209mmol), 2-bromoethyl methyl ether (35.3μl, 0.376mmol) and potassium carbonate (57.8mg, 0.418mmol) in acetone, react at 60°C for 12 hours, after the reaction Cool to room temperature, concentrate the reaction solution under reduced pressure, add distilled water, extract with ethyl acetate (50mL×3), wash with saturated brine, combine the organic phases, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure with column chromatography (Petroleum ether:ethyl acetate=1:2) separation and purification gave the target product (45 mg, reddish-brown solid, yield 40%). 1HNMR (600MHz, CDCl 3 )δ8.54(d, J=8.4Hz, 1H), 7.74(s, 1H), 7.68(s, 1H), 7.45(s, 1H), 7.26(d, J=8.5Hz, 1H), 6.07( d,J=7.2Hz,1H),4.45–4.36(m,1H),4.29–4.26(m,1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com