A method for preparing functionalized polyester by organocatalysis
A catalytic preparation and functionalization technology, applied in the field of chemistry, can solve the problems of low grafting rate and graft polymerization degree of glucan, and achieve the effects of good biocompatibility, production cost saving, and high polysaccharide grafting rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
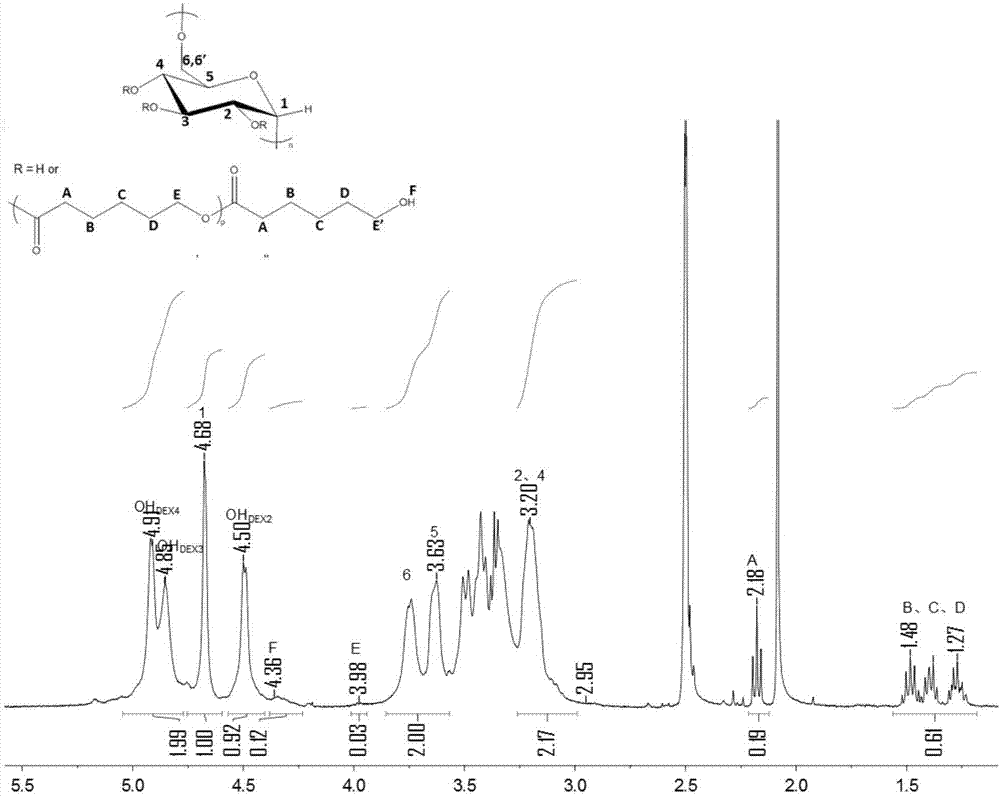
[0031] Example 1: Graft modification of dextran 5,000 catalyzed by p-toluenesulfonic acid for 2h at 50°C
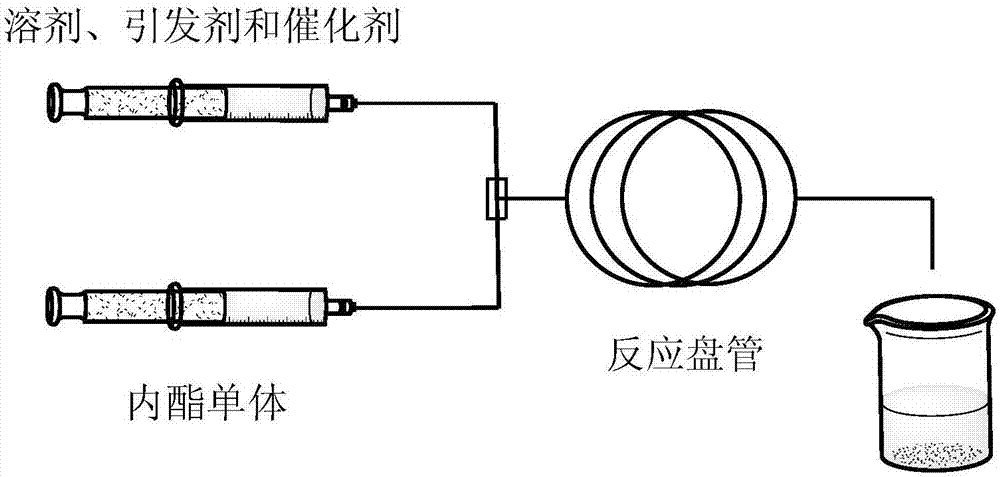
[0032] Weigh 5,000 5.0g of dextran and dissolve in 19ml of distilled water, add 0.72g of p-toluenesulfonic acid to the solution and stir the solution thoroughly, draw it up with a syringe and install it in the plunger pump a of the syringe after all the solids are dissolved.
[0033] The δ-valerolactone preserved in argon is connected to the Schlenk device under the protection of argon, and 8ml of δ-valerolactone monomer is extracted with a syringe, and the syringe is installed in the syringe plunger pump b. The specific gravity of the ester monomer and the solvent water is 0.3-0.5.
[0034] The specification of the microflow field reaction coil was selected as 16.30 m (diameter 0.5 mm), and the retention volume V=3.2 ml.
[0035] Set the calculated propulsion flow rates of the plunger pumps a and b respectively, connect the micro flow field reactor, heat the coil tube a...
Embodiment 2
[0041] Example 2: Graft modification of dextran 5,000 by methanesulfonic acid at 100°C for 1 h
[0042] Weigh 5,000 5.0g of dextran and dissolve in 35ml of distilled water, add 0.28ml of methanesulfonic acid to the solution and stir the solution thoroughly, draw it up with a syringe and install it in the syringe plunger pump a after all the solids are dissolved.
[0043]The ε-caprolactone stored in argon is connected to the Schlenk device under the protection of argon, and 9ml of ε-caprolactone monomer is extracted with a syringe, and the syringe is installed in the syringe plunger pump b. The specific gravity of the ester monomer and the solvent water is 0.2-0.3.
[0044] The specification of the microflow field reaction coil is selected as 6.36m (diameter 0.8mm), and the retention volume V=3.2ml.
[0045] Set the calculated propulsion flow rates of the plunger pumps a and b respectively, connect the microflow field reactor, heat the coil tube at 100°C, set the retention tim...
Embodiment 3
[0051] Example 3: Graft modification of dextran 10,000 by diphenyl phosphate catalyst for 1 h at 50°C
[0052] Weigh 10,000 5.0g of dextran and dissolve in 19ml of distilled water, add 1.0g of diphenyl phosphate to the solution and stir the solution thoroughly, draw it up with a syringe and install it in the syringe plunger pump a after all the solids are dissolved.
[0053] The δ-valerolactone preserved in argon is connected to the Schlenk device under the protection of argon, and 8ml of δ-valerolactone monomer is extracted with a syringe, and the syringe is installed in the syringe plunger pump b. The specific gravity of the ester monomer and the solvent water is 0.3-0.5.
[0054] The specification of the microflow field reaction coil was selected as 4.08m (diameter 1.0mm), and the retention volume V=3.2ml.
[0055] Set the calculated propulsion flow rates of the plunger pumps a and b respectively, connect the microflow field reactor, heat the coil tube at 50°C, set the ret...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com