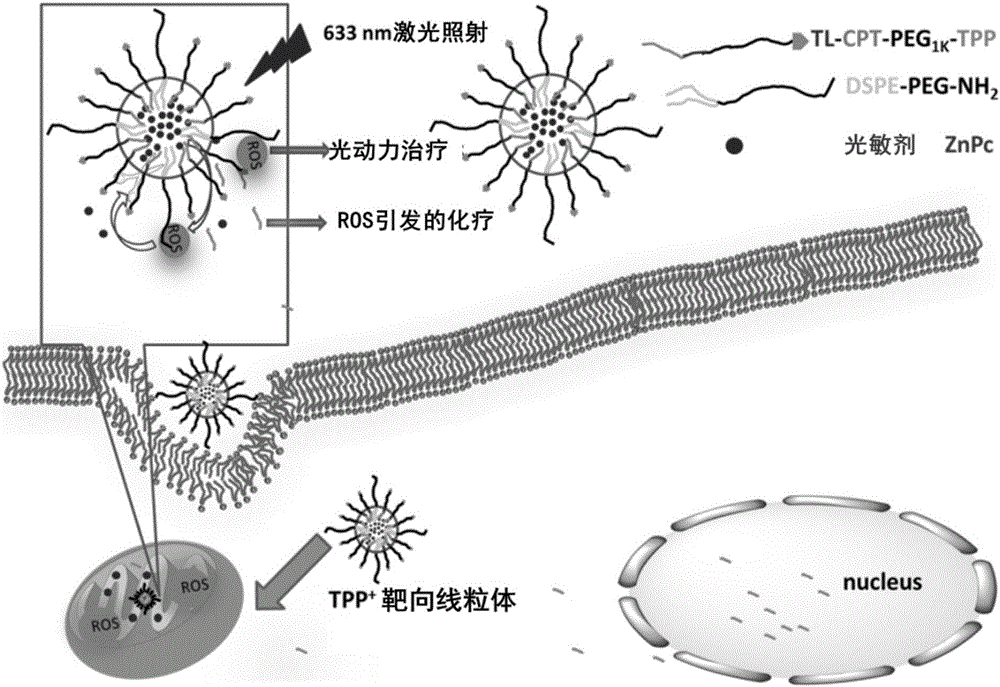
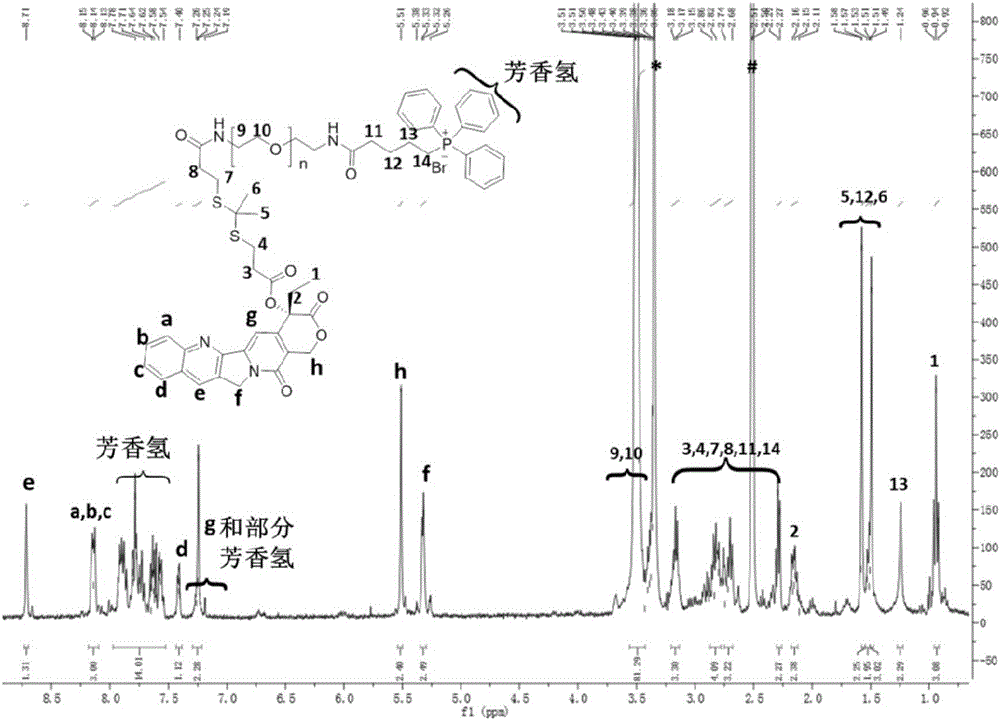
Mitochondrion-targeting nano-drug delivery system and preparation method and application thereof
A nano-drug and delivery system technology, applied in drug combinations, pharmaceutical formulations, medical preparations with non-active ingredients, etc., can solve problems such as few researches, and achieve good biocompatibility, good stability, and uniform particle size. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
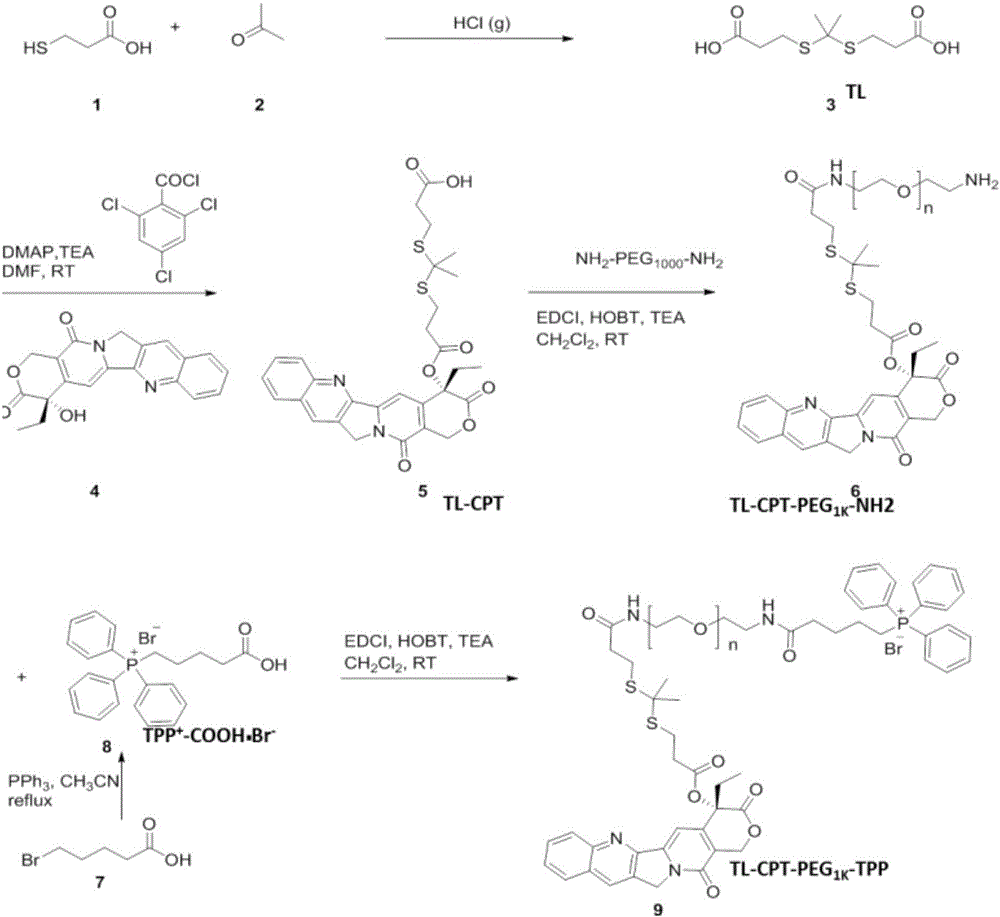
[0056] 1. The steps for synthesizing ROS-sensitive thioketal linker (TL) are as follows:
[0057] Anhydrous 3-mercaptopropionic acid (5.2g, 49.1mmol) and anhydrous acetone (5.8g, 98.2mmol) were saturated with dry HCl gas and reacted at room temperature for 4h. The reaction product was crystallized, filtered, washed with hexane and cold water, and vacuum freeze-dried with thiol linker to obtain the product.
[0058] 2. The experimental steps of synthesizing TL-CPT are as follows:
[0059] TL (252.1mg, 1.0mmol) was dissolved in anhydrous 10mL DMF, followed by adding triethylamine (TEA, 3.0mmol), 2,4,6-trichlorobenzoyl chloride (241.9mg, 1.0mmol), dimethyl Aminopyridine (DMAP, 24.4 mg, 0.2 mmol), stirred for 10 min. Camptothecin (CPT, 174.1 mg, 0.5 mmol) dissolved in 10 mL of DMF was then added, and the reaction was stirred at room temperature for 24 h. Quench the reaction with water and CH 2 Cl 2 Extract 5 times. The crude product was passed through a silica gel column to ...
Embodiment 2
[0076] 1. The synthesis steps of the ROS-sensitive thioketal linker (TL) are the same as in Example 1, and will not be repeated here.
[0077] 2. The experimental steps of synthesizing TL-DOX are as follows:
[0078] TL (252.1mg, 1.0mmol) was dissolved in anhydrous 10mL DMF, followed by adding triethylamine (TEA, 303.6mg, 3.0mmol), 2,4,6-trichlorobenzoyl chloride (241.9mg, 1.0mmol) , Dimethylaminopyridine (DMAP, 24.4 mg, 0.2 mmol), stirred for 10 min. Doxorubicin (DOX, 271.76mg, 0.5mmol) dissolved in 10mL of DMF was added, and the reaction was stirred at room temperature for 24h. The reaction was quenched with water, and the crude product was passed through a silica gel column to obtain pure product.
[0079] 3. The steps for connecting TL-DOX to the amino group at one end of polyethylene glycol are the same as those in Example 1, and will not be repeated here.
[0080] 4. The modification steps of triphenylphosphine are the same as those in Example 1, and will not be repea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com