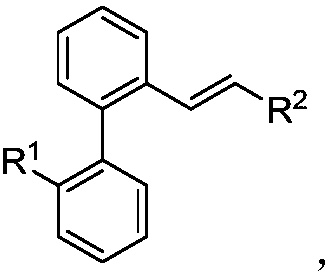
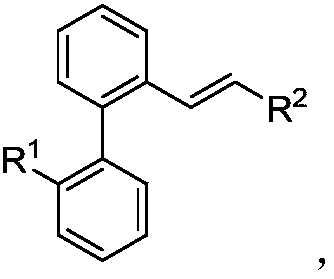
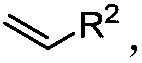Preparation method of difunctional biphenyl compound modified by alkyl and alkenyl
A technology of difunctionalized biphenyls, which is applied in the field of difunctionalized biphenyls and their preparation, can solve the problems of poor economic benefits, low efficiency, inability to difunctionalize, etc., and achieves simple operation, mild reaction conditions, The effect of reducing the generation of reaction waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Palladium-catalyzed 2-iodobiphenyl reacts with chlorinated alkanes and alkenes to synthesize butylstyryl-modified biphenyl compounds, including the following steps: add a stirring bar, 0.45mg Pd(OAc ) 2 (10mol%), corresponding 2-iodobiphenyl (0.2mmol), 105μL chlorobutane (1mmol), 23μL styrene (0.2mmol), 33μL IPA (2equiv), 78mg KOAc (4.0equiv), 138mg K 2 CO 3 (5.0 equiv) and 2.0 mL of DMF, and then use a nitrogen balloon to exchange 5 times to fill the reaction tube with nitrogen. Seal the reaction tube with a matching polytetrafluoroethylene stopper, and place it in a magnetic stirrer at 70°C for 12 hours. At the end of the reaction, the reaction tube was cooled to room temperature, the reaction solution was diluted with ethyl acetate and filtered through diatomaceous earth, the filtrate obtained after washing several times with ethyl acetate was concentrated with a rotary evaporator, and the obtained crude product was purified and separated by a silica gel plate. Th...
Embodiment 2
[0111] Palladium-catalyzed reaction of 2-iodobiphenyl with chlorinated alkanes and alkenes to synthesize butylstyryl-modified biphenyls:
[0112] In a 25mL Schlenk reaction tube, add a stir bar, 4.5mg Pd(OAc) 2 (10mol%), corresponding 2-iodobiphenyl (0.2mmol), 105μL chlorobutane (1mmol), 23μL styrene (0.2mmol), 33μL IPA (2equiv), 78mg KOAc (4.0equiv), 138mg K 2 CO 3 (5.0 equiv) and 2.0 mL of DMF, and then use a nitrogen balloon to exchange 5 times to fill the reaction tube with nitrogen. Then seal the reaction tube with a matching polytetrafluoroethylene stopper, and place it in a magnetic stirrer at 70°C for 12 hours. At the end of the reaction, the reaction tube was removed from the heating device and cooled to room temperature. The reaction solution was diluted with ethyl acetate and filtered through diatomaceous earth. The filtrate obtained after washing several times with ethyl acetate was concentrated with a rotary evaporator to obtain the crude product Purify and iso...
Embodiment 3
[0158] Mix 2-iodobiphenyl, bromo-n-butane, styrene, isopropanol, potassium acetate, potassium carbonate, and palladium acetate in a molar ratio of 1:3:1:1:2:2:0.01, and dissolve in the solvent N-methylpyrrolidone, and then exchanged 5 times with a nitrogen balloon to fill the reaction tube with nitrogen. Then seal the reaction tube with a matching polytetrafluoroethylene stopper, and place it in a magnetic stirrer at 50°C for 24 hours. At the end of the reaction, the reaction tube was removed from the heating device and cooled to room temperature. The reaction solution was diluted with ethyl acetate and filtered through diatomaceous earth. The filtrate obtained after washing several times with ethyl acetate was concentrated with a rotary evaporator to obtain the crude product The corresponding butylated product was purified and isolated by silica gel plate, and the yield was determined by weighing. The final product is:
[0159]
[0160] The yield was 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com