Method for preparing p-toluenesulfonyl isocyanate with carbonyl fluoride
A technology of toluenesulfonyl isocyanate and carbonyl fluoride method, applied in the preparation of sulfonic acid amide, organic chemistry, etc., can solve problems such as side reactions, production risks, and difficulty in product separation and purification, and achieve high yield and reduce pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
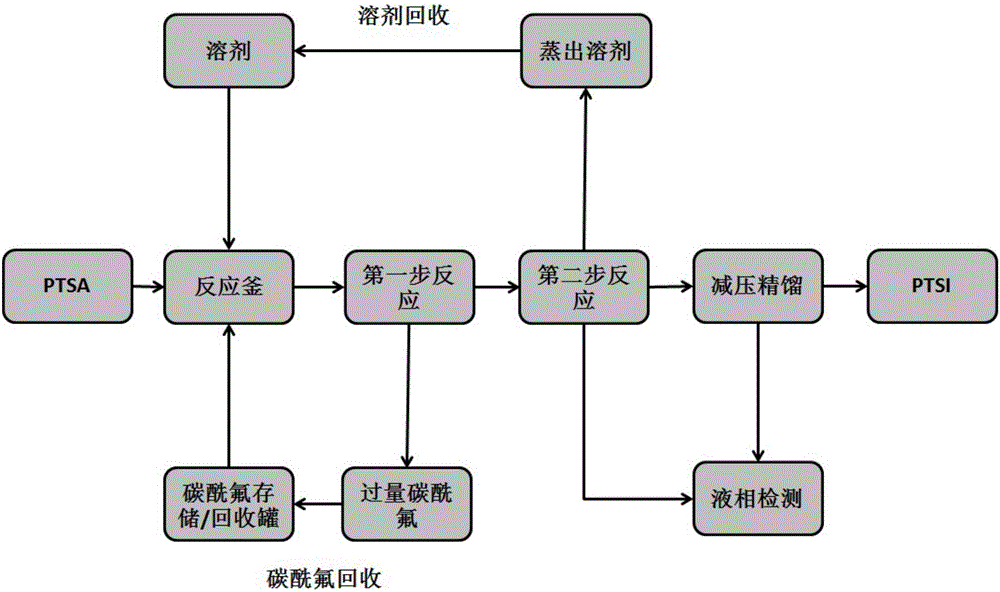
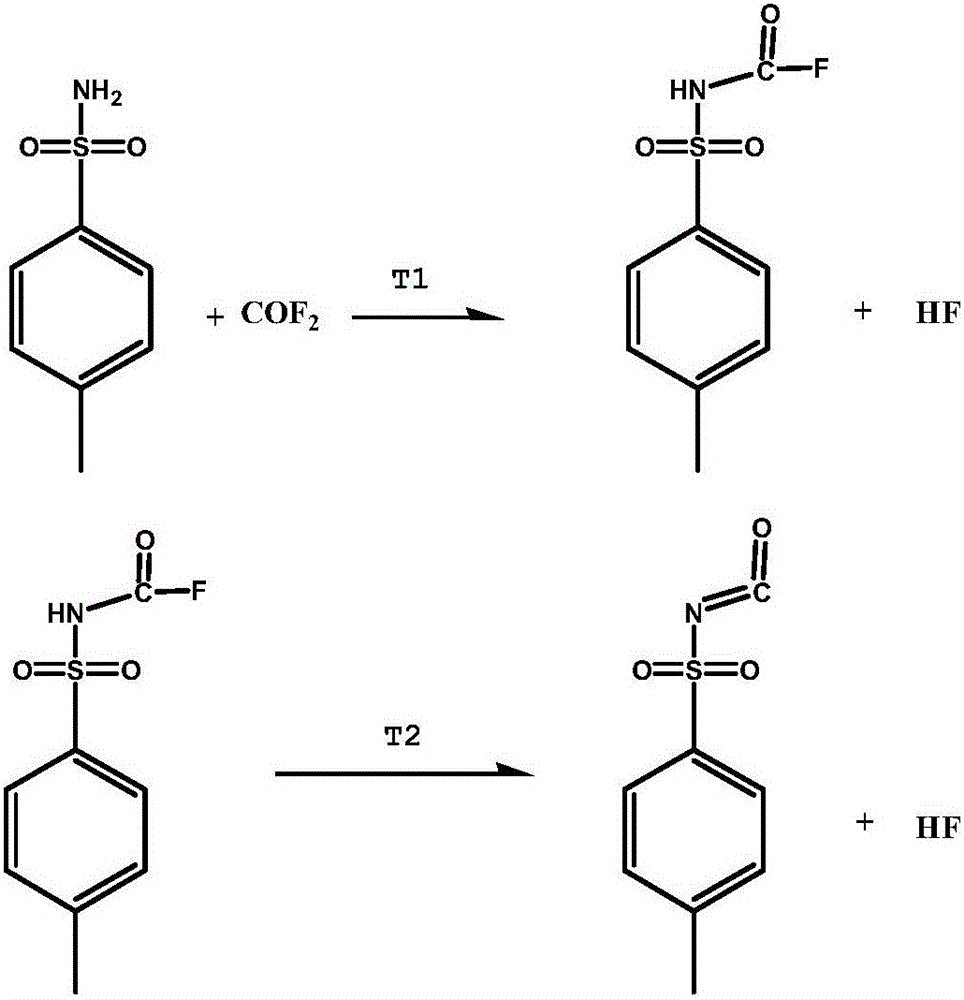
[0033] Add 25.6g (0.15mol) of p-toluenesulfonamide and 50ml of toluene into a 150ml stainless steel reaction kettle with stirring, fill in 28.0g of carbonyl fluoride, close the system, and react at 80°C for 3 hours, recover excess carbonyl after the reaction is completed Fluorine, then increase the reaction temperature to 140°C under normal pressure, react for 2 hours, and distill off the solvent toluene at the same time, the reaction is completed in 2 hours, and the crude product p-toluenesulfonyl isocyanate is 26.9g, the purity is 94.2%, and the reaction yield is 85.9%.
Embodiment 2
[0035] Add 25.6g (0.15mol) of p-toluenesulfonamide and 75ml of toluene into a 150ml stainless steel reactor with stirring, fill in 40.8g of carbonyl fluoride, close the system, react at 80°C for 3 hours, recover excess carbonyl after the reaction is completed Fluorine, then raise the reaction temperature to 140°C under normal pressure, react for 2 hours, and distill off the solvent toluene at the same time, finally get 28.0 g of p-toluenesulfonyl isocyanate as a crude product, with a purity of 95.6% and a reaction yield of 90.7%.
Embodiment 3
[0037] Add 25.6g (0.15mol) p-toluenesulfonamide and 50ml xylene into a 150ml stainless steel reactor with stirring, fill in 29.9g carbonyl fluoride, close the system, react at 80°C for 3 hours, and recover excess carbon after the reaction is completed Acyl fluoride, then increase the reaction temperature to 140°C under normal pressure, react for 2 hours, and distill off the solvent xylene at the same time to finally obtain 29.6 g of p-toluenesulfonyl isocyanate as a crude product with a purity of 95.0% and a reaction yield of 95.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com