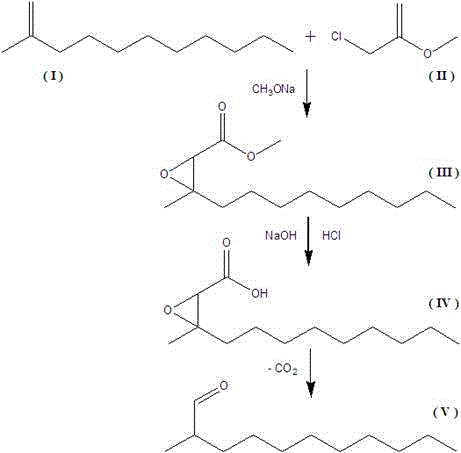
Modified synthetic method of methyl nonyl acetaldehyde intermediate
A synthesis method and technology of methyl nonyl ketone, applied in the field of chemistry, can solve problems such as reducing the rate of alkylation reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0015] Dissolve 7.6 g (0.14 mol) of sodium methoxide in 40 ml of cyclohexane, cool down to 5 °C under stirring, slowly add 17.3 g (0.1 mol) of methyl nonyl ketone (I) and 13.2 g of methyl chloroacetate (0.12 mol) the mixed solution of (II), the dropwise addition process is about 40 minutes, continue to keep warm for 5 hours (within the range of 4.5 to 5.5 hours), after the reaction is complete, add dilute hydrochloric acid to the reaction solution to terminate the reaction, adjust the pH The value is 4. After filtering to remove solid impurities, the organic layer is distilled under reduced pressure to recover cyclohexane and methyl nonyl ketone (I), which are separated by thin-layer chromatography and dichloromethane:petroleum ether=10:1 The lotion was rinsed and separated by silica gel column chromatography to obtain 3-methylepoxydodecanoic acid methyl ester (III), and the reaction yield reached 89.6%.
Embodiment 3
[0017] Dissolve 7.0 g (0.13 mol) of sodium methoxide in 40 ml of cyclohexane, cool down to 5 °C under stirring, slowly add 17.3 g (0.1 mol) of methyl nonyl ketone (I) and 13.2 g of methyl chloroacetate (0.12 mol) the mixed solution of (II), the dropwise addition process is about 40 minutes, continue to keep warm for 5 hours (within the range of 4.5 to 5.5 hours), after the reaction is complete, add dilute hydrochloric acid to the reaction solution to terminate the reaction, adjust the pH The value is 4. After filtering to remove solid impurities, the organic layer is distilled under reduced pressure to recover cyclohexane and methyl nonyl ketone (I), which are separated by thin-layer chromatography and dichloromethane:petroleum ether=10:1 The lotion was rinsed and separated by silica gel column chromatography to obtain 3-methylepoxydodecanoic acid methyl ester (III), and the reaction yield reached 87.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com