Production method of flumioxazin
A technology of profenachlor and a production method, applied in directions such as organic chemistry, can solve problems such as difficulty in preparing high-content products, unreported post-processing methods, complicated nitro group positioning, etc., and achieves solutions to large-scale production problems and production The effect of low cost and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
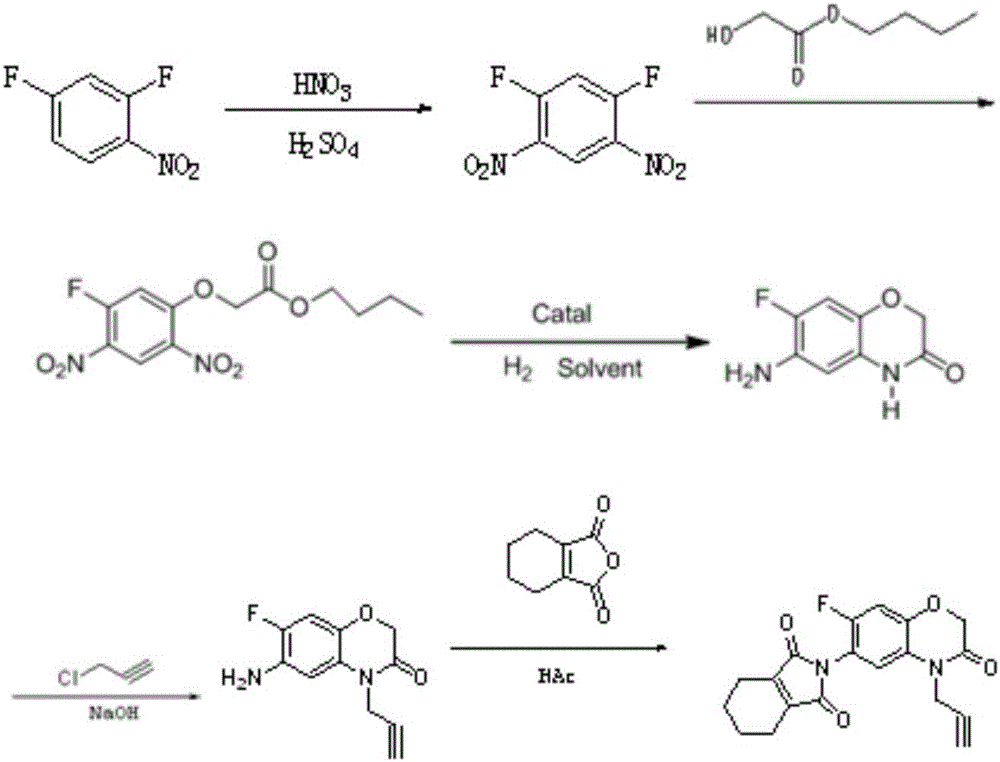
[0044] a pressfigure 1 The production method of the propargyl flurafen that shown reaction formula carries out, it may further comprise the steps:
[0045] (1) Nitrification reaction
[0046] Inject 42.5kg of fuming nitric acid and 280kg of 98% concentrated sulfuric acid into the reaction kettle, control the temperature at 20-30°C, then add 100kg of 2,4-difluoronitrobenzene dropwise under stirring conditions, and control the reaction temperature during the dropwise addition The temperature is 20-45°C. After the dropwise addition, the temperature is raised to 50°C and reacted for at least 1 hour. Then take 0.5mL of stirring liquid, add 0.5mL of dichloroethane, take the upper organic phase, wash it with water, and then detect it under the condition of liquid phase analysis. The mass content of 2,4-difluoronitrobenzene, when the mass content of 2,4-difluoronitrobenzene is less than 0.2%, add 200kg 1,2-dichloroethane to the reaction kettle for extraction and layering, The obtaine...
Embodiment 2
[0059] The difference between this example and Example 1 is: in step (2) etherification reaction, when the mass content of 1,5-difluoro-2,4-dinitrobenzene is less than 6%, the reaction system Add soft water to quench the phase separation, and the lower layer solution after phase separation uses NaHCO 3 Solution is carried out alkali washing, and other steps are identical with embodiment 1.
Embodiment 3
[0061] The present embodiment differs from Examples 1 to 2 in that: step (2) the lower layer solution after the etherification reaction is quenched and phase-separated uses Na 2 CO 3 Solution is carried out alkali washing, and other steps are identical with embodiment 1 or 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com